The other sulfides: Organic sulfur species in amine solvents
This article examines the behavior of carbonyl sulfide (COS), C1–C5 mercaptans (R1SH–R5SH), methyl ethyl sulfide (MES), dimethyl sulfide (DMS) and dimethyl disulfide (DMDS) in amine solvent systems. The objective here is to explore the current state of open industry knowledge in this area and compare it to gathered field test data. This article will highlight what is and what is not known and is a good starting point for future study on this topic, as not much field data has been previously published. The article contains:
- A review of the chemistry of organic sulfur species in amine systems
- Trending and analysis of key operating parameters for data from 50 industrial amine solvent absorbers
- A study of the organic sulfur challenge at a European refinery.
Optimizing the removal of organic sulfur species can eliminate the need for, or reduce the size of, downstream gas conditioning units in new facilities. In existing systems, this offers the advantage of extending cycle times on molecular sieves and reducing the consumption of caustic and the volume of mercaptide and disulfide waste streams requiring disposal.
Note: The removal of sulfide species from liquefied petroleum gases (LPG) through liquid-liquid extraction is not covered here. Carbon disulfide (CS2) is also not included in this study, as its presence in gas streams is thought to be mostly limited to tail gas treating units (TGTUs). The authors’ company has extensive field data for LPG treaters and CS2 removal, and this data will be the subject of future work.
Background. The non-hydrogen sulfide (H2S) sulfide species COS, C1–C5 mercaptans (R1SH–R5SH), methyl ethyl sulfide (MES), dimethyl sulfide (DMS) and dimethyl disulfide (DMDS) are frequently present in hydrocarbon streams in the oil, natural gas and biogas industries and are commonly called ‘organic sulfur.’ The behavior of organic sulfur species in amine systems is generally poorly predicted but can have a major impact on plant operations. This has been highlighted during work on two recent projects.
The first, an ultra-sour natural gas plant in the Middle East, contained ~1,000 ppmv of non-H2S sulfide in its feed. Units were built downstream of the amine absorber to remove the organic sulfur from the gas product. When the authors’ company commissioned and performed guarantee testing on the plant, it became apparent that the amine system was almost entirely removing the organic sulfur species from the gas. This effectively rendered the downstream mol sieve organic sulfur removal units unnecessary at start of run (SOR) operating conditions. Details on this project are provided by Schulte, et al.1
The second project was a European refinery, Preemraff Lysekil, where compliance with strict internal and governmental environmental standards on stack sulfur dioxide (SO2) emissions was limiting throughput. The refinery initiated an analytical testing program to determine the primary sources of the SO2 emissions. This was traced back to the treated gas from a specific amine absorber (one of the 12 onsite). Specifically, the visbreaker LPG fraction (which contains significant mercaptan sulfur) was being added into the feed to this absorber—when burned, this fuel gas accounted for a large portion of the refinery sulfur emissions. Similar emissions resulting from mercaptans have subsequently been found at four other refineries in Europe and three in the U.S., all of which also vaporize a large portion of the visbreaker/coker LPG fraction into their refinery fuel gas.
Both projects experienced a significant economic impact because the behavior of organic sulfur species in amine systems is not well-developed in industry. This article will present the information available to the industry and from the authors’ company, and highlight areas where work is needed to improve understanding.
The theory of organic sulfur species removal with alkanolamine solvents. In an amine absorber tower, the gas being treated is contacted counter-currently with an amine solvent. These solvents are alkaline in nature and remove target species—which are acidic in aqueous solutions—such as H2S and carbon dioxide (CO2) by chemical absorption. Non-dissociating species (e.g., hydrogen, nitrogen, methane) can also be partially absorbed in the amine solution through physical absorption, which is predominantly a function of pressure, temperature, residence time and solubility.
A good review of the available prior work on the removal of COS and lighter mercaptans in amine systems can be found in literature by Kohl and Nielsen.2 Reports on removal rates are given by other authors, but these are drawn from a very limited number of data points—in many cases, the results are from a single installation. These numbers are summarized in TABLE 1. To the authors’ knowledge, there is little information on heavier mercaptans (C4SH and higher), MES, DMS or DMDS in open literature.
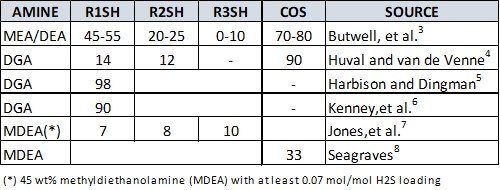 |
| TABLE 1. Percentage removal of various organic sulfur species by amines as reported in literature |
The following pathways for organic sulfur species removal in an amine system are possible:
- Physical absorption
- Chemical absorption (acid-based reaction)
- Direct chemical reaction with amine
- Direct chemical reaction with water (hydrolysis) for some species (COS and CS2)
- Condensation or absorption into a separate liquid hydrocarbon phase
- Conversion via reaction with other species in the gas or amine solvent solution.
With multiple organic sulfur species and multiple pathways in an already-complex system, many gaps exist in industry knowledge with respect to how, and how much of, an organic sulfur species will be removed in a particular amine system.
Physical absorption is driven by the difference in each species’ partial pressure in the hydrocarbon phase being treated and in the aqueous amine phase. Absorption will depend on temperature, pressure, contact time and solubility. Organic sulfur species are normally only present at ppm levels and pressure is thus not a significant driving force for their removal.
In addition to physical absorption, mercaptan sulfur species will also undergo chemical absorption, reacting with amine to form mercaptide salts. The resulting system is quite complex to model mathematically and has previously been described for R1SH–R4SH mercaptan sulfur by Jones, et al.7 The other organic sulfur species are not known to dissociate. Mercaptan competes with H2S and carbonic acid (H2CO3) for chemical absorption by amine molecules. Protonated amine bound to sulfide or carbonate is not available for reaction with mercaptans and reduces the driving force for their removal. Until large amounts of H+ ions are no longer being formed (once all the H2S and CO2 are removed), the mercaptan removal by chemical absorption will be poor.
In the case of COS, it is believed by many in the industry that a hydrolysis reaction is the primary mechanism for its removal.2 This reaction is shown in Eq. 1:
COS + H2O → CO2 + H2S (1)
A zwitterion reaction mechanism for COS with primary and secondary amines was proposed by Little, et al.9 in Eqs. 2 and 3:
COS + R2NH ↔ R2NH+COS- (2)
R2NH+COS- + (Base) ↔ R2NCOS- + (Base) H+ (3)
This two-step reaction mechanism is comparable to that of the absorption of CO2 by amines. It is comprised of a first step, where a COS-amine zwitterion is formed, and a second step, where the COS-amine zwitterion is deprotonated in the presence of a base (unreacted amine present in the solution).
For tertiary amines, Little, et al.10 proposed the following (Eqs. 4 and 5):
COS + MDEA + H2O ↔ MDEAH+ + HCO2S- (4)
HCO2S- + MDEA + H2O → MDEAH+ + HCO3- + HS- (5)
The first reaction of COS with methyldiethanolamine (MDEA) to form protonated MDEA and monothiocarbamate is reversible and an order of magnitude faster than the irreversible second reaction. The overall reaction is very similar to hydrolysis. A comparison of the reaction rates of various amines with COS, adapted from the thesis of Little11, is shown in FIG. 1.
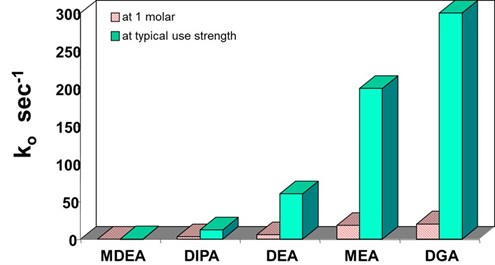 |
| FIG. 1. Pseudo-first order rate constants for reaction of amine solvents with COS.11 |
While prior research by Rahman, et al.12 found no evidence for the direct reaction of methyl mercaptan with monoethanolamine (MEA), diethanolamine (DEA), diglycolamine (DGA), diisopropanolamine (DIPA) or MDEA, COS can react directly with MEA, DEA, DGA and possibly DIPA to form degradation products. The rate of degradation with COS is significantly faster in primary (MEA, DGA) amines than in secondary and tertiary ones. In DGA, the degradation product [N, N bis(hydroxyethoxyethyl) thiourea abbreviated as BHEEU] is reversible in a reclaimer; therefore, COS degradation is primarily a concern in MEA systems where it converts MEA to diethanolurea, which is not reversible.
According to Dawodu,13 COS reacts with DEA to form MEA, bis-hydroxyethyl imidazolidone (HEI), bis hydroxyethyl piperazine (BHEP), hydroxyethyl oxazolidone (HEOD), tris-hydroxyethyl ethylenediamine (THEED) and bis-hydroxyethyl ethylenediamine (BHEED). Butwell3 reported that only 2% of COS in the feed gas reacted with DEA irreversibly, while removal of up to 80% is possible primarily through the hydrolysis reaction. DIPA is thought to degrade with COS, but the rate of reaction is slower than the time allowed for experiments to determine this.2 DIPA is considered a suitable solvent for COS removal in conjunction with a physical solvent (i.e., the sulfinol-D formulation). Rahman, et al.12 did not detect any reaction of COS with MDEA or dimethylethanolamine (DMEA).
While it is thought possible for COS to react directly with water, this reaction is too slow to be industrially relevant. Cantrell, et al.14 applied Danckwerts’ criteria15 to show that the reaction of COS directly with water is not relevant in terms of available contact time on amine absorber trays.
Both COS and mercaptan sulfur removal are disadvantaged by the presence of H2S and CO2 in the amine. Therefore, most of their removal is thought to be in the top part of an amine absorber after the bulk of the H2S and CO2 have already been absorbed. Following from this theory, some industry rules-of-thumb have evolved:
- The removal of organic sulfur only happens after most of the H2S and CO2 have been removed. Anecdotally, columns with more contact stages have reported better removal. An example can be found in literature.11
- Mercaptan and COS removal are favored by lower lean amine CO2 and H2S loadings. Therefore, more regeneration (reboiler duty use) will be required.
- Lighter mercaptans exhibit larger solubilities than heavier mercaptans in amine solutions. Heavier mercaptans are increasingly non-polar (more hydrocarbon-like) with an increasing carbon number, therefore less soluble in the highly-polar aqueous amine.
- Amine systems attempting to slip CO2 (tail gas treating and acid gas enrichment units) will not have good organic sulfur removal, as significant absorption of CO2 will be occurring in the top trays of the contactor, which will compete with organic sulfur removal.
Mercaptan has been shown to react with molecular oxygen in basic aqueous solutions under mild temperature and pressure conditions to form disulfides and peroxide or water.16 Uncatalyzed conversion rates are slow, but a wide range of simple metal salts can serve this purpose, including Fe2(SO4)3, CoCl2 and CuSO4, among others.17 This reactivity is the basis for the Merox processes developed by UOP used to sweeten a wide variety of hydrocarbon products, from LPG to jet fuel. While Merox units use advanced proprietary catalysts for efficient conversion, the simple metal salts commonly encountered in amine-based treating units from the process of corrosion may act in a similar manner, if the unit suffers from oxygen incursion.
Experimental work by Borgeson18 almost a century ago suggested that mercaptans could be formed from the reaction of H2S with some aldehydes, ketones, olefins, acetic and propionic acid. None of these experiments was done in an alkaline environment and mercaptan was detected only by odor due to the analytical limitations of the time.
Concurring with these observations, preliminary lab experiments by the authors’ company led to the detection of mercaptan in the gas headspace above aqueous amine solutions mixed with methanol or acetone after pressurizing with H2S gas and heating to temperatures approximating those encountered in an absorber tower. While further investigation of these results is required, they suggest it may be reasonable to observe a change in the ratios or even an increase in concentration of organic sulfurs between the outlet and inlet of an absorber under certain conditions.
The theory of organic sulfur phase partitioning. One difficulty in deciding which amine is best suited to remove particular organic sulfur species is the question of exactly how it will partition in the various liquid and vapor streams in a refinery or gas plant. One method of determining sulfur species partitioning is via vapor pressure comparisons to associated hydrocarbon streams. FIG. 2 shows a log-scale bar graph of vapor pressure, in descending order, for components that may be present in a feed hydrocarbon stream.
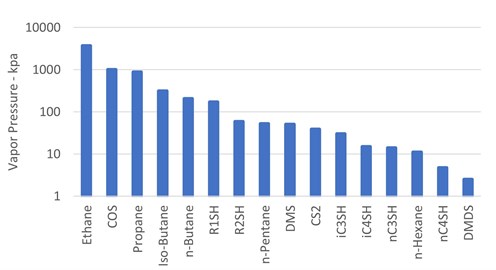 |
| FIG. 2. Vapor pressures of possible feed components at 21°C. |
As indicated by Likins and Hix,19 when considering vapor pressures only, the chart shows that COS should distribute into the propane stream and possibly into an ethane/propane mix stream. Methyl mercaptan (R1SH) should distribute primarily into the n-butane stream with some going with both the i-butane and gasoline streams. Ethyl mercaptan (R2SH), DMS and CS2 should end up primarily in the gasoline stream with only minor distribution into the n-butane stream. The other heavier sulfur compounds should end up almost entirely in the gasoline stream.
In practice, contrary to the vapor-pressure expectations of very little mercaptan partitioning into the propane stream, more than 50%–60% of the R1SH has been measured there. COS indeed partitions between the ethane/propane mix and the propane stream.
Simulators also attempt to calculate partitioning based on available property data (critical properties, eccentric factors, regressed VLE data, etc.), but even with these data, simulators are challenged to match one another in key component partitioning predictions, perhaps because not all property data are valid.
Presently, in-plant analysis with sulfur-specific gas chromatograph testing capabilities is the best way to understand the partitioning of sulfur species and, ultimately, the quantity of each component in each hydrocarbon stream that must eventually be removed by the amine solution. Feed-stream sulfur speciation and treated-stream sulfur speciation will characterize the true performance of the amine solvents in removing the various extended sulfur species in the plant.
FIELD TEST DATA AND TRENDS
Gas compositions used to determine the figures presented in the following section come from samples collected on live plant sites. All samples were collected in sulfur-inert containersa,b. Samples were analysed on proprietary gas chromatographsc (GCs) equipped with proprietary columnsd and flame photometric detectors (FPD). The GCs were transported to site and calibrated in-situ with a traceable standard for all reported components.
COS. To render the trends more meaningful, data points with very little COS (< 4 ppm) were removed, as these are more likely to be errors due to sampling and analysis. Cases where the amine solvent was significantly contaminated with another amine were also not considered. The data presented below come from five refinery fuel gas absorbers operating between 3.5 barg and 13 barg with inlet COS concentrations ranging from 4 ppmv–336 ppmv.
The removal of COS in DEA was trended against the following: absorber operating pressure, lean loading, rich loading, amine strength, amine temperature, simulated absorber maximum bulge temperature, inlet COS content and gas-to-liquid ratio. The removal of COS in DEA appears to have a strong linear correlation with the gas-to-liquid ratio, with other parameters having less impact. This is shown in FIG. 3.
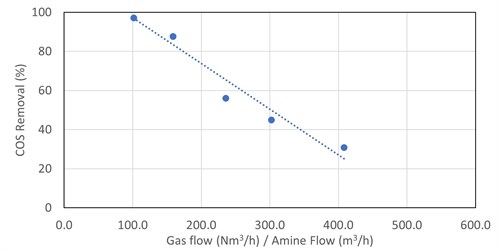 |
| FIG. 3. COS removal in DEA vs. absorber V/L ratio for five absorbers. |
For COS removal in MDEA, a linear correlation was found between the simulated temperature bulge in the absorber column and COS removal (FIG. 4). The data come from four refinery fuel gas absorbers, two TGTUs and two natural gas plant absorbers. The absorbers operated between atmospheric and 29 barg pressure, with the inlet COS ranging from 9 ppmv–522 ppmv. Absorber operating pressure, lean loading, rich loading, amine strength, lean amine temperature, inlet COS content and gas-to-liquid ratio did not show strong correlations (linear or otherwise) with COS removal.
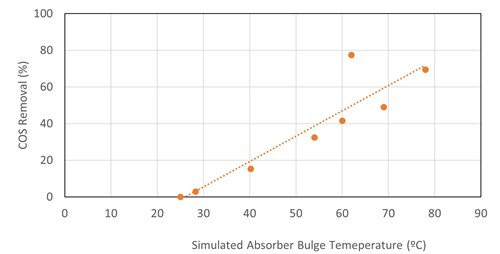 |
In TGTU absorbers, there is (generally) no significant temperature bulge in the absorber and amine flowrates are minimized to reduce CO2 absorption. Therefore, other factors may dominate COS absorption in TGTUs. Generally, not much COS is removed in TGTU absorbers.
R1SH. For primary and secondary amines, there was a very rough correlation between increased methyl mercaptan removal efficiency and smaller gas-to-liquid ratios (FIG. 5). Note: These are live plants and not controlled experimental environments. This was further investigated in a test run at Preemraff Lysekil on the T2904 visbreaker fuel gas absorber—a much stronger trend was observed, shown in FIG. 6. The removal of up to 80% of inlet mercaptan appears to be achievable with generic primary and secondary amines when low gas-to-liquid ratios are used in the column.
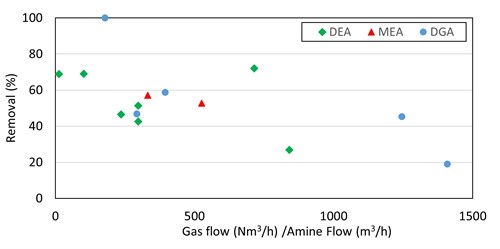 |
| FIG. 5. Methyl mercaptan removal in primary and secondary amines vs. absorber V/L ratio for 14 absorbers. |
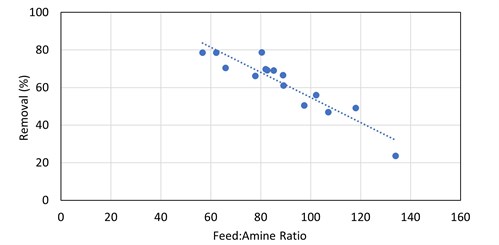 |
| FIG. 6. Methyl mercaptan removal in DEA vs. absorber V/L ratio for absorber T2904 at PreemRaff Lysekil. |
This trend has also been observed in literature. A paper presented by Dow20 describes an improvement in light mercaptan removal using an amine-only solvent blend when the gas-to-liquid ratio is decreased by ~40%. Further presentations by Dow and Spectra Energy21 present data from a test run on the two trains at the Spectra Jedney plant in Canada. These data show an increase in mercaptan removal efficiency with their hybrid amine and physical solvent with decreasing gas-to-liquid ratios and increasing numbers of contact stages. Additionally, work describing the industrial testing of hybrid amine-physical solvent by Total S.A.22 at its Lacq plant in France describes decreasing the gas-to-liquid ratio by ~25% to obtain improved removal of methyl mercaptan with a DEA-thiodiglycol (TDG) mix.
There was no clear correlation of methyl mercaptan absorption in MDEA with any process parameters; removing 20% of inlet methyl mercaptan in the seven absorbers was (generally) being studied (FIG. 7).
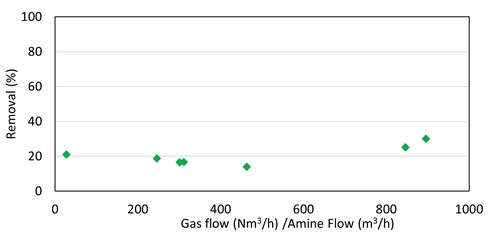 |
| FIG. 7. Methyl mercaptan removal by MDEA vs. absorber V/L ratio for seven absorbers. |
R2SH. For ethyl mercaptan absorption by DGA, the trend of higher removal at lower gas-to-liquid ratios was once again observed (FIG. 8). This trend was not seen with DEA or MDEA.
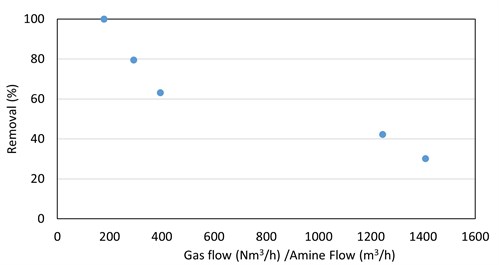 |
| FIG. 8. Ethyl mercaptan removal in DGA vs. absorber V/L ratio for five absorbers. |
Curiously, DEA and MDEA showed a trend of increased removal with decreased amine solvent strength (FIG. 9). This runs counter to conventional wisdom that higher amine strength will increase mercaptan removal. It is possible that ethyl mercaptan removal in these cases requires an initial dissociation reaction step into the water before reacting with the amine, which could explain why lower strength (large water content) solvent shows better removal rates. Salting out of the mercaptan salt or a mass transfer impact from the higher viscosity at higher strength could be potential explanations. Further investigation of this trend is required to draw valid conclusions.
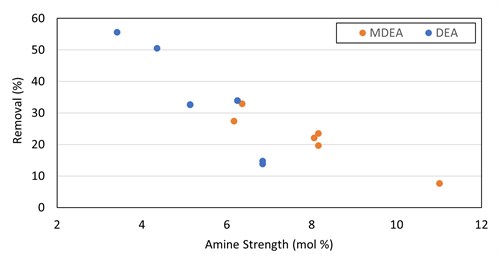 |
| FIG. 9. Ethyl mercaptan removal in DEA and MDEA vs. solvent strength. |
Other sulfur species. Significantly fewer data points are available for heavier mercaptans and other sulfides. The percentage removal of these species in various amines is given in TABLE 2. More data for these species are required before any correlation is meaningful, or for conclusions to be drawn.
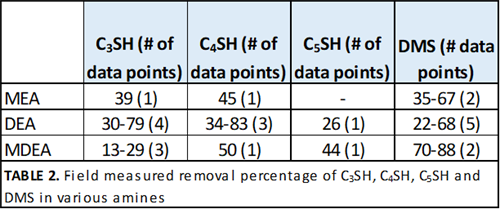 |
| TABLE 2. Field measured removal percentage of C3SH, C4SH, C5SH and DMS in various amines |
DMS was present in significant quantities in 9 of the 50 measured gas absorbers. In all instances, the gas in question was refinery fuel gas. No clear trends were observed and the number of data points per amine type were very limited. The removal of DMS ranged from 0%–88% efficiency with inlet DMS in sour gas ranging from 33 ppmv–784 ppmv.
There was not a large amount of statistically significant data for MES. In one instance, two absorbers in series using MEA with 38 and 35 trays, respectively, reduced the MES content from 87 ppmv to 73 ppmv, and then from 73 ppmv to 52 ppmv, respectively. This configuration is unusual and saw significant formation of DMDS (8 ppmv–89 ppmv) in the first 38-tray absorber—the science explaining this observation is not fully developed.
DMDS was (generally) not present in the gas inlet to the treaters. Two instances were found in addition to the situation mentioned above. In the first MDEA fuel gas absorber, operating at 6 barg, the DMDS concentration decreased from 18 ppmv to 8 ppmv across the treater. Secondly, in a DEA absorber operating at 6 barg, the inlet DMDS concentration decreased from 14 ppmv to 11 ppmv.
Simulated vs. actual removal with DEA. Three commercially available simulators were considered for their ability to predict organic sulfur removal from a fuel gas by generic DEA solvent. Simulators 1 and 2 had R1SH, R2SH, R3SH, R4SH, COS and DMS as available components, while Simulator 3 only had the capability to predict C1SH and C2SH removal and was therefore not included in this assessment.
The measured removal of the mercaptan species from a recent field test (not Preemraff Lysekil) was compared to the two model predictions (FIG. 10). From this and other industry experiences, it appears that current (2020) commercial simulators significantly underpredict the removal of organic sulfur species in generic amine systems.
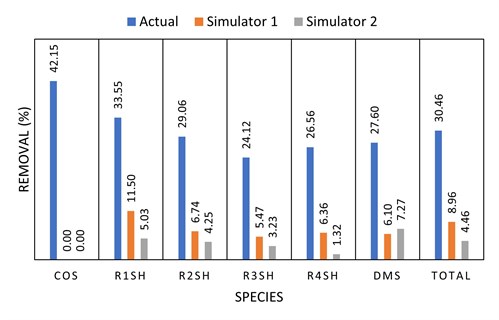 |
| FIG. 10. Simulated vs. actual organic sulfur removal in a DEA fuel gas absorber. |
Onsite optimization at Preemraff Lysekil. As part of the emissions minimization strategy at Preemraff Lysekil, an analytical test was conducted across the facility’s treating system to identify sources of sulfur emissions. Onsite testing eliminated the TGTU, as it was working exceptionally well and no tail gas from the Claus plant was leaking into the stack. Further analysis of the main amine system traced the source of the emissions to organic sulfur species in the fuel gas. These species were mainly entering the system via a DEA fuel gas absorber, which was also treating vaporized LPG from a thermal cracking unit (visbreaker). During the testing, some variations were detected in the inlet composition and the feed gas flowrate. The bulk of the sulfur leaving this column with the treated fuel gas was in the form of methyl and ethyl mercaptan.
Follow-up test runs were arranged to determine if the organic sulfur species in the fuel gas and, therefore, the SO2 emissions from the plant, could be reduced by operational changes. Adjustments to column temperature and pressure did not show appreciable results: ranges were limited by hardware considerations. Adjustment of the upstream cut-points on the visbreaker unit did, however, have a significant impact and sent more mercaptans and DMS to the light naphtha fraction rather than to the fuel gas absorber. The light naphtha fraction is subsequently hydrotreated, effectively converting the mercaptans and DMS to H2S, which can easily be absorbed in an amine treater.
The absorber was initially operating at low flood levels and, as such, did not have a high degree of contact between the amine solvent and the gas. The low contact was sufficient for the removal of H2S and COS, but the removal of the mercaptans was not optimal. Once the amine circulation was increased, the methyl mercaptan removal by the DEA solution increased from 24% to 78% (see FIG. 6). For ethyl mercaptan, there was a step-change increase from 15% removal to 40%–60% removal as the amine flow rate was increased to the point where there was adequate contact (FIG. 11). From that point, at subsequent smaller gas-to-amine ratios, there is no obvious correlation to the removal of R2SH.
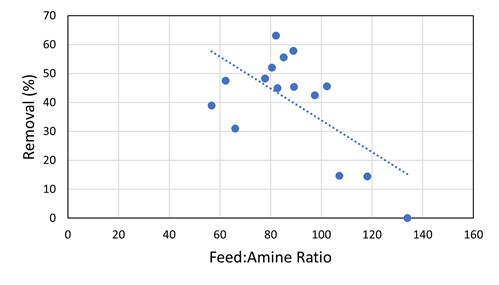 |
By adjusting gas-to-amine solvent ratios, it was possible to increase the removal of the organic sulfur species from 21% to 71% of the inlet concentration. The adjustment of upstream cut-points increased this further—the net result is that the organic sulfur species in the treated gas were reduced by 94% after field optimizations.
At the end of the test run, SO2 emissions associated with this absorber declined by more than an order of magnitude. Determining the source of the SO2 emissions and then optimizing process conditions during a dedicated test run, with testing and analytical support, significantly decreased the already-low emissions from the facility.
Takeaways. Field data reveal that generic amines are capable of better organic sulfur removal than historical literature or commercial simulators now predict.
In one instance, DGA was able to achieve 100% removal of the COS, CH4S, C2H6S and C3H8S, albeit at low gas-to-solvent rates, which is not beneficial for plant CAPEX and OPEX. Removal of CH4S and C2H6S by DGA correlated with gas-to-solvent ratios, with lower ratios showing higher rates of removal. Other operating parameters investigated did not appear to have a major impact for DGA.
DEA data show a correlation between COS and CH4S removal and gas-to-solvent ratio, as well as an unusual correlation between C2H6S removal and lower solvent strength. Removal of up to 100% of COS, 80% CH4S and 60% C2H6S was observed on some systems.
Absorption of COS by MDEA appeared to be linked to the temperature bulge within the absorber, with 80% absorption of COS by MDEA being the maximum observed. MDEA showed low absorption of methyl mercaptan (20%–30%) with no obvious correlation between the amount of absorption and other process parameters.
For ethyl mercaptan removal, the data showed an interesting trend of better removal of ethyl mercaptan with declining amine strength for MDEA and DEA, which could potentially be explained by a reaction mechanism in which the ethyl mercaptan reacts with the water initially, or viscosity effects.
For propyl, butyl and pentyl mercaptan, removal in the range of 20%–80% was observed in the field results, but there were insufficient data to establish links with process parameters or to draw significant conclusions. The removal of DMS ranged from 22%–88%, with MEA/DEA/MDEA capable of removing 70% under certain process conditions.
In some instances, significant reductions in organic sulfur in treated gas are possible by optimizing process conditions. This is best done through onsite analytical testing, as both the fundamental understanding of the behavior of organic sulfur species and the simulation of this behavior need improvement.
Further research is needed to improve the industry’s ability to predict (and hence optimize) organic sulfur species removal in amine systems. Specifically, improvement in simulator prediction capabilities is required. This will potentially require the generation of new or better experimental data for the solubility and absorption of organic sulfur species in amine systems on which simulator predictions are based. Also of interest is the formation chemistry of some organic sulfur species in amine systems (e.g., mercaptan formation from sour amine in the presence of methanol) and potential routes for DMDS formation. GP
ACKNOWLEDGEMENT
The authors would like to thank Preem AB for granting permission to publish data from its Lysekil facility.
NOTES
a Tedlar® bags
b Sulfinert® cylinders
c Agilent 6890 or 7890 gas chromatographs
d Agilent GasPro columns
LITERATURE CITED
- Schulte, D., S. van Wagensveld, C. Graham and B. Lynch, “DGA treatment of ultra-sour gas,” SOGAT Conference 2017, Abu Dhabi, UAE, March 27–30, 2017.
- Kohl, A. and R. Nielsen, “Gas purification,” 5th Ed., Gulf Publishing Co., Houston, Texas, 1997.
- Butwell, K. F., D. J. Kubek and P. W. Sigmund, “Alkanolamine treating,” Hydrocarbon Processing, March 1982.
- Huval, M. and H. van de Venne, “DGA proves out as a low-pressure gas sweetener in Saudi Arabia,” Oil & Gas Journal, August 17, 1981.
- Harbison, J. L. and J. C. Dingman, “Mercaptan removal experiences in DGA sweetening of low-pressure gas,” Gas Conditioning Conference, University of Oklahoma, Norman, Oklahoma, 1972.
- Kenney, T. J., A. R. Khan, P. E. Holub and D. E. Street, “DGA agent shows promise for trace sulfur compound removal from hydrocarbon streams,” GIU Sulfur Recovery Conference, May 15–17, Austin, Texas, 1994.
- Jones, C. E., N. A. Hatcher and R.H. Weiland, “Mercaptan removal from gases by absorption into amines and caustic,” Brimstone Sulfur Conference, Vail, Colorado, September 2013.
- Seagraves, J., “Sulfur removal in amine plants,” Hydrocarbon Engineering, December 2001.
- Little, R. J., G. F. Versteeg and W. P. M. Van Swaaij, “Kinetics study of COS with tertiary alkanolamine solutions. 1. Experiments in an intensely stirred batch reactor—Amines with primary and secondary amines in aqueous solutions,” Industrial and Engineering Chemistry Research, May 1992.
- Little, R. J., G. F. Versteeg and W. P. M. Van Swaaij, “Kinetics of COS with primary and secondary amines in aqueous solutions,” AIChE Journal, February 1992.
- Little, R. J., "Selective carbonyl sulfide removal in acid gas treating processes,” University of Twente dissertation, Enschede, the Netherlands, 1991.
- Rahman, M. A., R. N. Maddox and G. J. Mains, “Reactions of carbonyl sulfide and methyl mercaptan with ethanolamines,” Industrial and Engineering Chemistry Research, April 1989.
- Dawodu, O. F., “Degradation of diethanolamine solutions by carbonyl sulfide and carbon disulfide,” PhD thesis, University of British Columbia, Vancouver, B. C, Canada,1991.
- Cantrell, J., G. McIntyre, C. Daniels and E. Stewart, “Operational considerations of side reactions in gas sweetening systems,” Laurance Reid Gas Conditioning Conference, Norman, Oklahoma, February 27, 2017.
- Danckwerts, P. V., Gas-liquid reactions, Mc-Graw-Hill Book Co., New York, 1970.
- Wallace, T. J., A. Schriesheim, H. Hurwitz and M. B. Glaser, “Base-catalyzed oxidation of mercaptans in the presence of inorganic transition metal complexes,” Industrial & Engineering Chemistry Process Design and Development, Vol. 3, July 1964.
- Murray, F. E. and A. C. Harkness, “Oxidation of methyl mercaptan with molecular oxygen in aqueous solution,” Atmospheric Environment, Vol. 4, July 1970.
- Borgeson, R. W., “Reactions of organic compounds with liquid hydrogen sulfide,” Retrospective theses and dissertations 14212, Iowa State University, Ames, Iowa, 1928.
- Likins, W. and M. Hix, “Sulfur distribution with commercial simulators,” 46th Annual Laurance Reid Gas Conditioning Conference, Norman, Oklahoma, March 3–6, 1996.
- Bendell, S. A., J. M. Griffin and J. J. Lambrichts, “Current and future gas treating solvent technologies for improved mercaptan removal,” European GPA Amsterdam Conference, Amsterdam, Holland, August 2008.
- Lemonds, A., J. McJannett, P. Wissner and D. Majid, “Field operational data for hybrid solvent treatment of mercaptans in unconventional natural gas,” 26th GPA-GCC 2018 Annual Conference, Oman, March 2018.
- Lallemand, F., D. Roquet and C. Weiss, “Development and industrial validation of a new hybrid solvent for improved mercaptan removal,” 87th Annual GPA Convention, Grapevine, Texas, March 2–5, 2008.
 |
PHILIP LE GRANGE is a Chartered Chemical Engineer who has optimized, troubleshot, commissioned and trained staff on more than 100 amine solvent systems across 31 countries. Together with Mike Sheilan and Ben Spooner, Mr. le Grange is an author of the industry reference textbook, Amine Treating and Sour Water Stripping.
 |
KAIYR TEKEBAYEV is a Senior Process Engineer at Amine Experts specializing in the commissioning and startup of amine and sulfur facilities.
 |
FRANCIS LEBLANC heads up Amine Experts research and innovation initiatives and holds a PhD in chemistry from the University of Calgary.
 |
MICHAEL SHEILAN is the Co-founder of Amine Experts and a recognized authority in the amine industry. For 42 yr, he has provided training, consulting and technical expertise in amine treating and gas dehydration.
 |
MARCUS ADOLFSSON is a chemical engineer with extensive experience as the Amine and Sulfur Plant Operations Engineer at both the Preems Lysekil and Preem Gothenburg refineries.
 |
DANIEL YARNOLD is a Chemical Engineer at Preems Lysekil with more than 10 yr of industrial experience.
 |
GILLES THEVENET has 38 yr of experience in the oil and gas industry and provides troubleshooting expertise at Preem Lysekil.




Comments