Technical and economic impacts of piperazine content in MDEA solvents
Amine units used in gas and liquid hydrocarbon treating utilize various solvent formulations. Some are amine-based, and some are not. These blended formulations can be open-knowledge or proprietary formulas from a number of different amine suppliers. Usually, these formulations are selected based on a review of the plant design, feed acid gas content (CO2 and H2S) and treated gas requirements. In the authors' experience, a considerable number of amine units over-treat or, conversely, are strained to meet specification with the solvent in place. Further evaluation of these units has revealed that modifications to the solvent formulation may result in more ideal treating conditions.
This article discusses the effects of non-ideal amine formulations and the opportunities for reduced costs and improved treating conditions made by reformulating the solvent. A particular case is studied where a formulated methyl diethanolamine (MDEA) solvent was over-treating. An evaluation of several formulations with reduced piperazine concentration was performed to identify formulations that would potentially reduce solvent cost and rich CO2 loading.
Amine unit background. The amine solvent at a gas plant amine unit (FIG. 1) comprised 50% water, 47% MDEA and 3% piperazine. Piperazine is a cyclic diamine used to improve CO2 removal in MDEA-based solvent formulations. The plant had little trouble meeting specifications for CO2 content within the range of conditions observed and the formulation in use, but the rich CO2 loading estimated in the system was above recommended guidelines. The plant engineers wanted to explore a potential reduction in piperazine content in future solvents to reduce amine solvent costs and rich amine CO2 loading.
 |
| FIG. 1. Amine system evaluated for solvent reformulation. |
The plant was processing up to 90 MMsft3d of natural gas, but generally bypassing up to 20 MMsft3d of gas to reduce operational costs while still meeting a 2.5% specification for CO2 content in the treated gas. The plant normally operates with an amine circulation rate of 190 gal/min (GPM); the design circulation rate is 250 GPM. The operator wanted to decrease piperazine concentration in the amine solvent to reduce both solvent costs and rich loading while staying just under the 2.5% specification. A conditional evaluation was performed to understand if a potential reformulation was feasible and, if so, what conditions must be modified.
The process was evaluated under several sets of conditions with varying MDEA/piperazine formulations to determine the lowest piperazine concentration required to meet specifications. This article summarizes the evaluation work performed, the results and interpretations, and the recommendations for solvent reformulation.
Process simulation overview. A rate-based simulator packagea was used to evaluate the amine system because of its ability to accurately predict the actual operating conditions and performance of amine gas treating systems. The software package uses a mass transfer rate-based model for column calculations in the amine contactor and regenerator, as opposed to equilibrium stage models used in other simulators. The rate-based model delivers a more accurate prediction of system operation and performance because it takes into account the actual design of the absorber column, as well as its internals.
Equilibrium stage models, by contrast, do not incorporate column design details into the simulation but instead rely on empirical data to estimate reaction kinetics. Equilibrium stage models use ideal stages for column calculations, which do not account for actual tray or packed-bed designs and do not provide a predictive output. The assumptions made for column designs in an equilibrium stage model will be accurate only with empirical data provided for that case, so any modification to the model input will not be simulated accurately without empirical data provided for the new conditions.
The objective of the evaluation was to predict operational conditions and performance of the amine system with different amine formulations. The rate-based model was necessary to provide accurate predictions in the absence of any empirical data for potential new formulations. Empirical data for the existing formulation was used to build a base model. That output matched with the empirical data for the treated gas, thereby validating the model accuracy.
The Deshmukh-Mather thermodynamic model is used by the software to predict vapor-liquid equilibrium behavior in the system, which is based on the Debye-Huckel theory regarding the behavior of electrolytic solutions. This model predicts the complex interactions involved in amine treating more accurately than conventional equilibrium models.
However, a thermodynamic model alone will not accurately predict amine system operation and performance. A truly predictive model of amine system operation and performance must account for five effects occurring in an absorber or regenerator:
- Mass and energy balance around phases on each tray (or packing section)
- Thermodynamic phase equilibrium (this model must be activity-based for the components present and must include an aqueous electrolyte model accounting for chemical reactions)
- Interfacial equilibrium between phases
- Chemical kinetics affecting mass transfer rates
- Mass and heat transfer for components and energy moving across interfaces.
FIG. 2 depicts several aspects involved with acid gas absorption in an amine absorber. The absorption of acid gas components into the amine phase (Ni) depends on the acid gas concentration in both phases relative to the interface (Pi – Pi* and Ci* – CiL), the film mass transfer coefficients (kg and ki0) and the enhancement factor (Ei). The mass transfer coefficients depend on equipment properties such as tray/packing design, fluid properties, and hydraulics. The enhancement factor depends on reaction kinetics and takes diffusion with reaction into account.
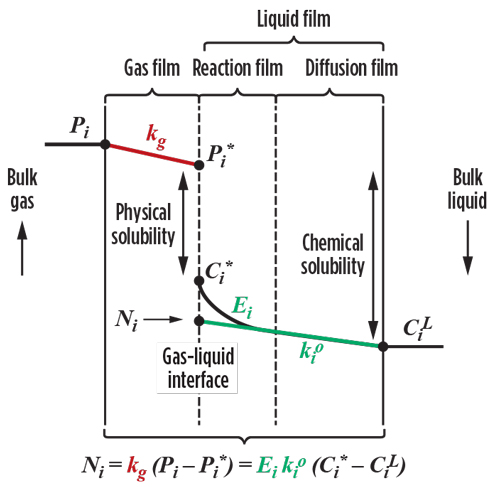 |
| FIG. 2. Diagram representing the various effects contributing to absorption in an amine contactor.b |
Equilibrium stage models account only for mass and energy balances (but only around ideal stages) and thermodynamic phase equilibrium; generally, they do not include an adequate aqueous electrolytic model. Interfacial equilibrium, chemical kinetic effects on mass transfer, and mass and heat transfer for components crossing interfaces are not accounted for.b
Simulation design and conditions. To simulate the system properly, normal and worst-case operating conditions for the feed gas and lean amine were collected from historical data. The conditions used for this project are shown in TABLE 1.
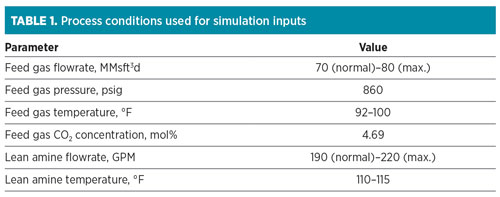 |
The full composition of the feed gas was also provided and used in the simulation. In addition, several design drawings and schematics of the amine contactor were collected by plant personnel, and information on the sizing and tray design was incorporated into the simulation. Parameters such as the number of trays, tray spacing, tower diameter, tray and valve type, and tray active area were used as inputs for the simulation.
The simulation PFD is shown in FIG. 3. The feed gas composition and process conditions were input into the feed gas block; the amine formulation, amine analysis results and process conditions were input into the lean amine block; and the tower internals information was input into the contactor block. The simulation was then performed, and output data for the rich amine, treated gas and gas-to-pipeline streams were calculated. A feed gas flowrate prior to bypass of 90 MMsft3d was used for the simulation with a bypass flowrate of 10 MMsft3d–20 MMsft3d.
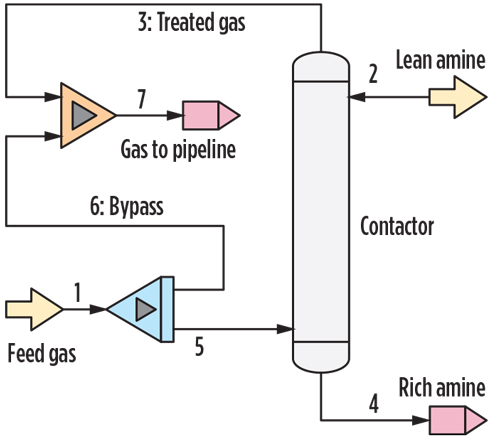 |
| FIG. 3. Process flow diagram of the amine system evaluated. |
Results and interpretation. Several different scenarios were simulated, using the program, by varying three key parameters:
- Piperazine concentration
- Feed gas flowrate
- Lean amine flowrate.
The objective of the study was to determine if a combined gas-to-pipeline CO2 specification of 2.5% could be achieved at lower piperazine concentrations, even at worst-case conditions. Piperazine concentrations were varied from 1.5%–3% in different scenarios.
The lean amine flowrate was also varied in each scenario to potentially facilitate a more efficient treating process. In some cases, an increase in amine flowrate can reduce rich loadings to within recommended guidelines (0.5 mol/mol) to prevent corrosion. In other cases, a decrease in amine flowrate can reduce the reboiler duty while maintaining treated gas CO2 content below specification. The feed gas flowrate to the amine absorber was varied between 70 MMsft3d and 80 MMsft3d, but 70 MMsft3d was targeted to reduce rich loading and reboiler duty.
TABLE 2 shows the results of each scenario that was simulated. The data includes the variable inputs used for each run, as well as the key performance indicators predicted by the simulation output. Values in red were not within recommended guidelines or required specifications.
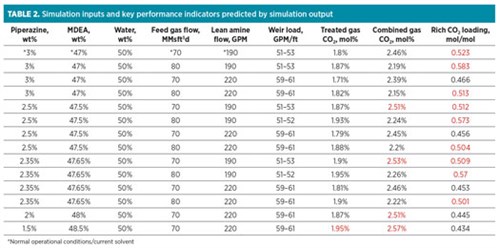 |
The normal operational conditions and current solvent were first simulated to validate the accuracy of the model. The treated gas CO2 content predicted by the simulator matched very closely with that measured at the plant during the same conditions (1.8%). The close match between simulated and actual conditions showed that the model was accurate and that inputs could be modified to reliably simulate real changes at the plant.
The normal operating conditions were also evaluated to understand if the unit could potentially be operated more effectively with the same solvent. The predicted rich loading at base conditions was above the recommended maximum, so the amine flowrate was increased to reduce it. This resulted in higher CO2 removal and increased reboiler duty, but the reduced rich loading should reduce corrosion. At 80 MMsft3d, the gas-to-pipeline CO2 specification was still met easily, but the rich loading could not be reduced adequately, even at the higher amine flowrate. This evaluation showed that (1) some corrosion risk is present with the current solvent at normal conditions that can be avoided by increasing amine circulation, and (2) some corrosion risk will inevitably be present at 80 MMsft3d with the current solvent.
The most aggressive scenario simulated was using 1.5% piperazine. This simulation was first performed at a 70-MMsft3d feed gas flow to the contactor and a 220-GPM lean amine flow to understand if enough CO2 could be removed at the higher bypass rate. The higher bypass rate allows for lower rich loading and is, in general, easier on the amine unit; therefore, lower bypass rates should be avoided when possible. Since the CO2 content was well over 2.5% in the gas to pipeline, even at increased amine flow, a higher level of piperazine was determined to be necessary. At 2% piperazine, the CO2 specification was again barely missed in the gas to pipeline.
At 2.35% piperazine, the CO2 specification was met in the gas to pipeline at 2.46%. Other scenarios were then performed for this formulation. It was determined that at the current amine flowrate, this formulation would not meet the CO2 specification unless the bypass flowrate was reduced. If the bypass flowrate was reduced, however, the rich loading would increase to above that at present conditions with the present solvent; this essentially showed that this formulation was only feasible and advantageous if the amine flowrate could be increased; otherwise it was more advantageous to remain at 70 MMsft3d to the contactor with the present formulation.
At 2.5% piperazine, the same scenarios were performed as with the 2.35% formulation, and similar results were observed. The amine flowrate needed to be increased for this formulation to work effectively at the higher bypass rate and provide benefit through reduced rich loading.
With the assumption that the amine flowrate could be increased, worst-case scenarios with amine concentration changes, feed gas temperature changes and lean amine temperature changes were then simulated to understand the ability of these solvent formulations to withstand difficult conditions. Scenarios were initially run at higher and lower amine concentrations, since amine is lost over time and must be made up. It was found that treating efficiency decreases at lower concentrations, so several scenarios were performed at 52% water concentration.
TABLE 3 shows the same tests performed as in TABLE 2, but with the total amine concentration reduced by 2% to consider situations where amine is lost and has not yet been made up. It was determined that both the 2.35% and 2.5% formulations were still able to meet CO2 specification at reduced amine concentration.
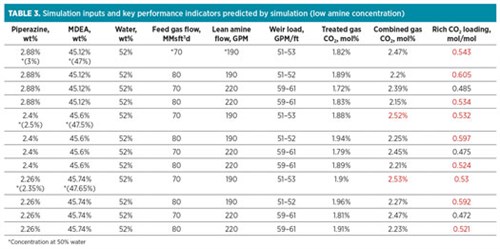 |
To further simulate non-ideal conditions for these formulations, the feed gas and lean amine temperatures were also varied. Scenarios were run with these changes at the higher bypass rate and increased amine flowrate, which would provide the most benefit for the unit overall. These cases were also performed at reduced amine concentration to consider the worst-case scenario.
TABLE 4 shows that at elevated feed gas temperature and reduced amine concentration, the 2.35% formulation would not meet CO2 specification (unless the bypass flowrate is increased). The 2.5% formulation was able to meet specification at elevated feed gas temperature, but only if the amine temperature is maintained; at elevated amine temperature and the other non-ideal conditions specified, the 2.5% formulation just barely misses specification. At the same worst-case conditions, the 3% formulation still met specification at 2.45% CO2.
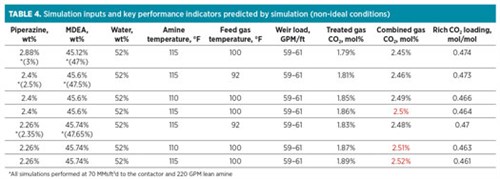 |
Economics and recommendations. A number of potential improvements were discovered in the evaluation results. It was primarily recommended to increase the amine flowrate to ≥ 220 GPM if corrosion in the rich amine circuit was of concern. This would reduce rich loading to levels of acceptable corrosion risk. In addition, the weir load on the trays was low at normal operating conditions, just above the minimum guideline of 50 GPM/ft. Spray flow can occur at low weir loads, leading to reduced treating efficiency and solvent carryover, but an increase in amine circulation could resolve the issue.
The best formulation and conditions for further consideration were chosen by finding a balance between adequate CO2 removal and rich loading. While several scenarios met specification, most did so at the edge of the high CO2 specification. The simulations with 2.35%–2.5% piperazine yielded the most ideal results in that both loading and CO2 content could be maintained comfortably below recommended maximums if the amine flowrate could be increased. Some worst-case conditions emerged where the 2.35%–2.5% formulations did not meet specification at the higher, 20-MMsft3d bypass rate; however, in those situations, the specification can be met by slightly reducing the bypass rate. It follows, then, that more efficient treating would be possible with a reduced piperazine concentration during normal conditions, but at non-ideal conditions the current formulation would work more effectively (to a certain extent).
It was recommended that a formulation with approximately 2.35% piperazine be used if the amine flowrate could be increased to 220 GPM. If the amine flowrate could not be increased, then a 3% formulation would be necessary. The economic implication of reducing the content of piperazine in MDEA solvents based on present market values was, on average, $0.1/lb–$0.15/lb ($0.9/gal–$1.3/gal) of solvent for each 1% reduction in piperazine. Reducing the piperazine concentration in the present solvent from 3% to 2.35% implied a total savings of $0.57/gal–$0.86/gal of MDEA solvent, or $2,860–$4,290 for a 5,000-gal inventory. (Note: the data is based on access to market price values).
This study and others have shown that plants can benefit from an evaluation of their amine solvent in place, especially as feed gas compositions change over time. Several standard solvents are sold to plants, but an ideal formulation—prepared based on thorough simulation and evaluation of the amine unit design and operating conditions—is implemented less frequently. Plant personnel should reassess, at least yearly, whether or not such an investigation should be performed with their current amine solvent, as a proper evaluation may reveal opportunities for reduced solvent costs, operating costs, energy usage, corrosion and other cost-effective performance improvements. GP
NOTES
a ProTreat process simulator package
b Based on ProTreat technical literature
 |
SCOTT WILLIAMS is a Process Engineer at Amine Optimization. He has industry experience in a number of projects in oil and gas, petrochemical, chemical and water treatment applications. As part of the Amine Optimization engineering group, Mr. Williams is responsible for technical design and solutions development in engineering and technology applications. He also provides support for analytical and specialized service projects. His recent work has been focused on amine unit contamination control, process stability and energy reduction. Mr. Williams holds a BS degree in chemical and biological engineering from the University of Colorado at Boulder.
 |
CODY RIDGE is a Lead Process Engineer at Amine Optimization Company and a Chemical Engineer from Texas Tech University in Lubbock, Texas. He is responsible for field engineering and technology development with Amine Optimization. Mr. Ridge has worked as an operator and process engineer in the Permian Basin.
 |
DAVID ENGEL has more than 25 yr of industrial experience in a variety of chemical engineering and chemistry areas. He is the inventor in 21 US patents and the author of a number of technical and scientific papers. Dr. Engel has developed business and technology for Eastman Kodak, Eli Lilly, Pentair, General Electric and Sulphur Experts worldwide. He has specialized in chemical engineering, process chemistry, optimization and contaminant removal technologies. He is the Managing Director of Nexo Solutions Companies. He holds a BS degree in industrial chemistry, a MS degree in chemistry and a PhD in organic chemistry. He is also Six Sigma and Project Management certified. Dr. Engel is a Committee Member of the American Filtration Society, Southwest Region; and a member of the Gas Processors Association Technical Section M, in addition to a member of the boards of directors at several companies.




Comments