Efficient acid gas removal using membrane systems—Part 1
With increasing demand for more energy, stranded natural gas reserves that would have been marginal or unprofitable years ago are now being developed. The main challenge in today’s natural gas treating plants is to remove high concentrations of carbon dioxide (and sometimes sulfur components) from sour gases produced from these remote or offshore stranded gas fields to meet stringent export specifications.
A number of methods are available for the removal of acid gases. However, membrane-based systems offer some clear advantages and opportunities for the efficient removal of high fractions of acid gases from these fields. The authors focus on the use of membrane technologies to make a large bulk cut of the acid gases, as well as to remove some mercaptans from raw natural gas. They also address hybrid membrane-amine systems, in which the amine process is used to perform the final purification.
Natural gas, which consists mainly of methane (CH4) and light hydrocarbons, may also contain acid gases, such as hydrogen sulfide (H2S) and carbon dioxide (CO2). In addition to acid gases, natural gas may comprise other sulfur contaminants, such as mercaptans, carbonyl sulfide (COS) and carbon disulfide (CS2). In addition to lowering heating value, acid gases can cause corrosion to carbon steel natural gas processing equipment and pipelines if free water is present. Combustion of these sulfur compounds produces sulfur oxide (SOx) air pollutants that must be limited to protect the environment and to prevent health problems.
CO2 is an inert gas that does not have any heating value, but it is the main source of greenhouse gas.1 Pipeline specifications may require the removal of acid gases to low levels. The added cost to remove such contaminants decreases the financial outcome of natural gas exploration and production. CO2 and H2S in the sour feed gas streams must be removed to specific levels to meet different application requirements (typically < 4 ppmv H2S and < 2% CO2 for pipeline-quality gas, and < 50 ppmv CO2 for LNG). Additionally, COS, mercaptans and other organic sulfur species that contribute to sulfur emissions must typically be removed to 30 ppm total sulfur.
A number of processes are available to remove acid gases from natural gas streams. Some are suitable for bulk acid gas removal. Others are effective for deep CO2 and H2S removal, but are ineffective in the removal of organic sulfur compounds such as mercaptans, disulfides or COS. The choice of technology, or combination of technologies, is dependent upon the needs of the gas processing plant owner, where the selection of an acid gas removal process can have a significant impact on project economics, especially when the feed gas contains a high percentage (e.g., > 15%) of acid gas. Amine absorption is the state-of-the-art acid gas removal technology, unless the acid gas concentration is rather high (resulting in high capital and operating costs). Therefore, the need exists for efficient systems to treat highly sour gases. Much research has been focused toward this goal.
One efficient method for the bulk removal of acid gases is separation via highly selective, permeable polymeric membranes. Until recently, most of the commercial membrane units have dealt with CO2 separation, rather than H2S separation. Membrane systems for bulk CO2 removal from natural gas are a mature technology, where they provide an efficient option for CO2 removal from natural gas, especially in remote and offshore locations.2 They are extremely adaptable to various feed gas flowrates, CO2 contents and product gas specifications. They are also relatively easy to start up and operate, with minimum weight and compactness for space efficiency. Membranes are also efficient in removing bulk H2S, as long as the removal requirements are not very stringent and the rejected, enriched H2S stream can be disposed.
In general, membranes are a bulk acid gas removal technology, and very low product specifications cannot be economically achieved.3 If the product specification is very tight, then another technology (e.g., amine washing) may need to be employed. A significant reduction in capital and operating costs may be achieved by using a hybrid membrane-amine system. This option is discussed in the sections below.
Traditional membrane technology (glassy membranes). Membrane systems, which consist of semipermeable elements (polymeric membranes), separate gases by selective permeation of the gas constituents. The gases dissolve in the membrane materials and move across the membrane barrier under a partial pressure gradient, which is established by maintaining a high feed pressure on one side of the membrane and a low pressure on the permeate side.
Many different types of membranes, classified by the materials of construction (i.e., glassy or rubbery polymers), have been developed for gas separation.
For CO2 separation from natural gas, the industry’s primary membrane materials have been cellulose acetate and cellulose triacetate, which are characterized as glassy polymers. These membranes are of the solution-diffusion type, and consist of a thin layer of cellulose acetate (or triacetate) on top of a thicker layer of a porous support material. In these membranes, the support layer and the separation layer are the same material and primarily differ in the relative thicknesses, and they are characterized as asymmetric. The active separation layer of these membranes is thin to maximize mass transfer, and the active layers require a supporting layer for mechanical strength.4
Membranes are formed either as flat sheets or hollow fibers. The flat sheets are typically combined into a spiral-wound element, while the hollow fibers are combined into a bundle. In either case, the cylindrical product is easily handled and ranges from 8 in.–12 in. or more in diameter, with the length of each element/bundle at approximately 4 ft.
Both membrane element types are used in the bulk removal of acid gas, and membrane vendors provide products that maximize the advantages of each type. The spiral configuration is widely used for acid gases, offering low permeate pressure resistance and tending to have lower per-bundle cost. Hollow-fiber modules offer a higher packing density, resulting in the need for fewer elements, but usually at a higher cost per element. Pressure drop in the bore of the hollow fibers can result in higher contaminant partial pressure in the permeate, reducing overall mass transfer driving force. However, the flow over the fibers can be more turbulent, leading to improved mass transfer on the retentate side of the membrane. With the infinite number of process conditions experienced in acid gas removal, the optimum membrane configuration may not be immediately obvious.
Membranes are asymmetric, as previously described, where a dense, thin separation layer is co-extruded with a more permeable (but thicker) support layer; or, alternatively, a composite two-part membrane where a porous support layer is coated with a dense separation layer. While each has advantages, the composite membrane allows the use of more specialized and expensive polymers for the separation layer. Since the volume of polymer used for the thin separation layer is small, the cost of the specialized separation layer is usually of limited concern. Such composite membranes are commercially supplied for both the spiral-wound and hollow-fiber designs.
Much research focus is on the separation layer, since this component provides the performance of the membrane. The support layer is also critical since it comprises the large majority of the membrane. Different support layers can tolerate liquid water, vapor phase or condensed heavy hydrocarbons, aromatics and acid gases to differing extents. When membranes fail in service due to impurities, then the support layer or the separation layer, which can have a different tolerance for impurities, can be damaged. For these reasons, the operating condition and impurities of a given application should be well defined so that the proper membrane material can be chosen. The membrane tolerance and lifetime can then be anticipated.
Mechanism of membrane separation. Polymeric membranes separate by solution and diffusion. In glassy membranes, the smaller and more soluble components (such as water, CO2 and H2S) permeate from high pressure to low pressure more quickly, leaving behind the less-soluble components (such as CH4, heavier hydrocarbons and nitrogen at high pressure).
The driving force across the membrane is the partial pressure differential of the permeating components. Therefore, as CO2 or H2S acid gases permeate across the membrane, the permeate-side acid gas concentration increases, while the feed/product-side acid gas partial pressure decreases as the feed gas moves toward the retentate outlet. The result is that bulk removal of acid gases is easily accomplished, while removing acid gases to low levels requires more membrane area. Since more membrane area (higher CAPEX) allows more hydrocarbons to permeate, operation at high CO2 removal levels or low CO2 product levels results in increased hydrocarbon loss.
The preferable operating conditions are: (1) high feed gas pressure, since it offers a higher acid gas partial pressure for a given component concentration; and (2) low permeate pressure (lower acid gas partial pressure). In most cases, the feed gas pressure is set by available well pressures or by required pipeline pressures. If the feed gas is available at a lower pressure than the pipeline requirement, compression will typically take place prior to membrane separation to take advantage of the higher pressure. In addition to the component partial pressures, the performance depends on the permeability and selectivity of the membrane elements for the different compounds.
The relative permeation of different compounds for a typical glassy membrane is illustrated in Fig. 1. Note that the acid gas components (CO2 and H2S) are moderately fast (end up in permeate) relative to the slower CH4 (which remains at high pressure in the retentate). Here, it is shown that CO2 is slightly faster than H2S (which is typical), although some glassy membranes have similar permeability of these components.
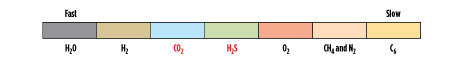 |
| FIG. 1. Relative permeation rates in glassy membrane. Source: Air Liquide. |
Rubbery membranes for acid gas separation. In recent years, rubbery membranes have been commercialized where the composite separation layer uses a polymer with properties that are quite different from the traditional glassy membrane. In general, glassy polymers follow the relative permeation rates in Fig. 1, while rubbery membranes can have more variable properties. For example, rubbery membranes are used for dewpoint adjustment and fuel gas conditioning. Heavy hydrocarbons dissolve in the rubbery polymer and permeate from high pressure to low pressure.5 The performance of glassy membranes is driven in part by the size of the permeating molecule, while, in rubbery membranes, performance is more dependent on the solubilities of the permeating molecules. This difference in the separation parameters can result in different permeation rates, as shown in Fig. 2. Note: H2S is shown as faster than CO2, and is indeed faster than shown for a glassy membrane.
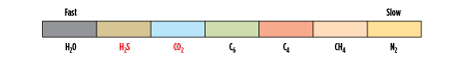 |
| FIG. 2. Relative permeation rates in a rubbery membrane that limits heavy hydrocarbons removal. Source: Air Liquide. |
This fast rate of H2S, and its high selectivity as compared to CH4, are used commercially to provide higher performance than would be achieved in a glassy membrane. The unique properties of rubbery membranes can offer advantages, but the desired application must be understood. Example options include:
- A membrane tailored to remove the acid gases of H2S and CO2 while leaving heavy hydrocarbons at high pressure (Fig. 2).
- A membrane that, in addition to removing the acid gases of H2S and CO2 and leaving some heavy hydrocarbons at high pressure, also removes the heaviest hydrocarbons to simultaneously adjust the dewpoint of the high-pressure product. The relative permeabilities of this type of membrane are shown in Fig. 3.
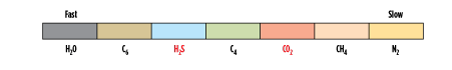 |
| FIG. 3. Relative permeation rates in a rubbery membrane that permeates some heavy hydrocarbons. Source: Air Liquide. |
It should be noted that rubbery membranes can be combined with glassy membranes in an optimized solution. For example, in a rich gas feed, where a glassy membrane can have a reduced life due to heavy hydrocarbons, a rubbery membrane can be used to remove the first cut of acid gases, along with some heavy hydrocarbons. This would be followed by a second-stage glassy membrane, where acid gases are further removed. Such a system is being built by one company, where the second-stage membrane is a maximum selectivity membrane, protected from heavy hydrocarbon condensation by a first-stage rubbery membrane.
Mercaptans removal with rubbery membranes. Rubbery membrane separations are driven by the solubility of the gas component, and mercaptans are quite soluble in certain rubbery membranes. For example, rubbery membranes can be used to permeate not only H2S but also a portion of mercaptans, if present. Such a separation is not perfect and the mercaptans do not “go away,” but they are largely removed and concentrated into the low-pressure permeate stream. This ability to permeate mercaptans gives the NGL plant designer another tool to address mercaptans where they may occur, such as treating raw gas, temperature swing adsorption (TSA) regeneration gas, or NGL streams where mercaptans can concentrate.
Acid gas permeability and selectivity for glassy and rubbery membranes. As a general rule for glassy membranes, CO2 permeation and the relative selectivity to CH4 follow a fairly consistent trend regardless of the polymer used. Therefore, while certain polymers offer benefits, the differences are somewhat limited for CO2/CH4 selectivity. Within the many glassy polymers available, H2S permeability is quite variable. A membrane supplier with a family of membranes, or a process licensor, may have more freedom to optimize the acid gas treatment process. The different performance of rubbery membranes adds another tool to the membrane supplier’s portfolio. Overall, where a membrane supplier has a portfolio of membranes that includes glassy and rubbery fibers, optimization using the available products offers a level of performance previously unattainable with membranes.
Acid gas removal description. With a semipermeable film of polymer, membranes utilize a solution-diffusion-based separation process to selectively dissolve CO2 and H2S into the surface. These components then diffuse across the polymer and support layer into a low-pressure permeate stream. H2S specifications may be difficult to meet, owing to the low mass transfer driving force (i.e., partial pressure) available at the required low H2S product concentrations (typically 4 ppm). In this respect, a need exists to develop novel membranes where the chemistry of the membrane results in preferential permeation of H2S and other sulfur-bearing components over hydrocarbons.6
The attributes of CO2 and H2S require the polymeric membranes to possess enhanced mechanical and chemical properties to endure flows and corrosion effects. Also, the toxicity of H2S has kept the number of relevant studies low, so few published findings are available. Most work done in this area is still in the laboratory, and not at commercial scale. One company has provided rubbery membranes for several plants with H2S feed levels around 4%, removing it to 100 ppm–1,500 ppm (with further downstream treatment as needed). Nevertheless, H2S removal by membranes is still evolving.
Membrane separation is best applied to bulk removal of acid gases, and where the rejected permeate can be used as fuel since membranes can permeate a significant amount of hydrocarbon. Where H2S is the targeted acid gas, disposal well reinjection is ideal since the permeate will generally contain too high a level of hydrocarbons to allow use in a Claus sulfur recovery unit. Hydrocarbon losses can be significantly reduced by using two-stage processing, where the permeate of the first stage is compressed and routed to a second stage for hydrocarbon recovery. While such a system can achieve high hydrocarbon recovery rates—higher than that of an amine plant, if fuel to the reboiler is accounted for—it does require the addition of a compressor and a second membrane stage.
Part 2 of this article, to appear in the July/August issue, will discuss membrane process configurations and technology selection. GP
Acknowledgment
Thanks are due to Scott Northrop for reviewing this manuscript and providing useful comments.
Saeid Mokhatab is a world-class expert in the natural gas processing industry. He has worked on the design and operation of several gas processing plants, and has contributed to gas processing technology improvements through 300 technical papers and two well-known handbooks published by Elsevier in the US.
Michael Mitariten is a Senior Director within the Advanced Technologies Group of Air Liquide in Woburn, Massachusetts. He is responsible for the membrane equipment and technology provided to the natural gas and biogas markets for gas purification to pipeline quality. He holds 20 patents and has published numerous papers in the field of gas separation. He is registered as a Professional Engineer in the state of New York.
Literature cited
- Mokhatab, S., W. A. Poe and J. Y. Mak, Handbook of Natural Gas Transmission and Processing, 3rd Ed., Gulf Professional Publishing, Burlington, Massachusetts, 2015.
- Cnop, T., D. Dortmundt and M. Schott, “Continued development of gas separation membranes for highly sour service,” 3rd Sour Oil and Gas Advanced Technology Conference (SOGAT 2007), Abu Dhabi, UAE, May 1–2, 2007.
- Echt, W. and M. Singh, “Integration of membranes into natural gas process schemes,” 87th GPA Annual Convention, Grapevine, Texas, March 2–5, 2008.
- Baker, R. W., “Future directions of membrane gas separation technology,” Ind. Eng. Chem. Res., Vol. 41, Iss. 6, 2002.
- Mokhatab, S., S. Northrop and M. Mitariten, “Controlling the hydrocarbon dewpoint of pipeline gas,” Petroleum Technology Quarterly, 3Q 2017.
- Hale, P. and K. Lokhandwala, “Advances in membrane materials provide new solutions in the gas business,” 83rd GPA Annual Convention, New Orleans, Louisiana, March 14–17, 2004.
- Anderson, C. L., A. Siahaan and K. Neumann, “Case study: Membrane CO2 removal from natural gas, Grissik Gas Plant, Sumatra, Indonesia,” 84th GPA Annual Convention, San Antonio, Texas, March 13–16, 2005.
- Cnop, T., “Membrane Technology Introduction,” GPA Europe Young Professional’s Training Session on Acid Gas Removal, Berlin, Germany, May 23, 2012.
- Echt, W., “Hybrid systems: Combining technologies leads to more efficient gas conditioning,” 52nd Annual Laurance Reid Gas Conditioning Conference, Norman, Oklahoma, February 24–27, 2002.
- Koch, D. R., W. R. Buchan and T. Cnop, “Proper pretreatment systems reduce membrane replacement element costs and improve reliability,” 84th GPA Annual Convention, San Antonio, Texas, March 13–16, 2005.
- Peters, R. and A. Jariwala, “Removing gas contaminants that affect CO2 removal membrane performance,” GPA Europe Spring Conference, Copenhagen, Denmark, May 25–27, 2011.
 |
Saeid Mokhatab is a world-class expert in the natural gas processing industry. He has worked on the design and operation of several gas processing plants, and has contributed to gas processing technology improvements through 300 technical papers and two well-known handbooks published by Elsevier in the US.
 |
Michael Mitariten is a Senior Director within the Advanced Technologies Group of Air Liquide in Woburn, Massachusetts. He is responsible for the membrane equipment and technology provided to the natural gas and biogas markets for gas purification to pipeline quality. He holds 20 patents and has published numerous papers in the field of gas separation. He is registered as a Professional Engineer in the state of New York.




Comments