Comparative study of DEA and MDEA treatments of acid gas
Egypt’s consumption of natural gas for all end uses (residential, industrial, commercial and power generation) has increased rapidly due to the growth of new markets, the use of natural gas in the production of petrochemicals and fertilizers, and rising demand for LPG. To satisfy growing domestic natural gas demand, Egypt has diverted its natural gas supply away from exports and is leaning on LNG imports to address the shortfall in consumption.
Natural gas must be treated, or “sweetened,” before it is fit for consumption by end users. Hydrogen sulfide (H2S), carbon dioxide (CO2) and water must be removed. Improvements in the efficiency and cost of gas sweetening processes are ongoing. Recent developments have focused on different kinds of adsorption and absorption methods, including dry adsorption and wet absorption.1
Methods for contaminants removal. Many treatment methods are available for the removal of H2S and CO2 from natural gas. One method uses a single chemical solvent, such as diethanolamine (DEA), methyl diethanolamine (MDEA), diglycolamine (DGA), diisopropylamine (DIPA), piperazine (PZ), monoethanolamine (MEA), sulfolane, or triethylamine (TEA). Another method uses a mixture of chemicals, such MDEA + PZ or MDEA + sulfolane.1 A strong focus has been directed on single amines—namely, DEA and MDEA.
This study examines the costs and efficiencies of DEA and MDEA for acid gas treating, using a proprietary simulation software program.a The study was carried out at an industrial gas sweetening plant operated by Gulf of Suez Petroleum Co. (GUPCO) in Ras Shukeir, Egypt.
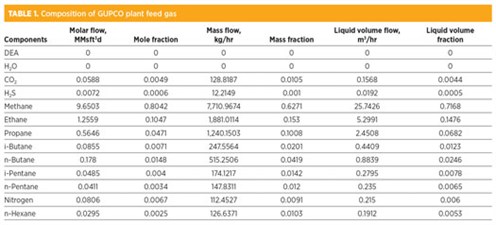
The feed gas used in the CO2 and H2S capture study has the following composition:
- Flowrate = 12 MMsft3d
- Temperature for rich acid gas = 41°C
- Pressure = 49 kg/cm2.
A detailed, rate-based model was developed for the two amine solutions in a water blend, using the simulation program to assess both process and economic performance. The study results showed that DEA removed 100% of H2S and 99.07% of H2S and CO2 from acid gas. MDEA removed 99.95% of H2S and 68.85% of both H2S and CO2.
Phases of gas treatment. Gas containing H2S can be divided into two categories:2 acid gas and sour gas. Natural gas containing H2S or other sulfur compounds is considered sour, while gas with only CO2 is considered sweet. Acid gas has high contents of CO2 and H2S, while sour gas contains moderate amounts.3
High concentrations of H2S in pipelines, tanks and separators can corrode equipment and can, in some cases, cause hazards to workers. H2S content in natural gas is limited to 4 ppm.2 CO2 is also corrosive to equipment and reduces the Btu value of gas. The normal accepted content of CO2 in untreated natural gas is 4 mol%–5 mol%.4 Both acid gas and sour gas also contain small amounts of other sulfur compounds—e.g., thiols (CSH) and carbonyl sulfide (COS). After removal of these contaminants, natural gas becomes suitable for use.
When a gas stream is added to an absorption column with a relatively high flowrate, the corresponding amount of amine will be relatively low. Water is added, up to 75 mol%, to amine gas sweetening solutions to ensure good contact between the natural gas and liquid and to increase the efficiency of the sweetening process.5,6
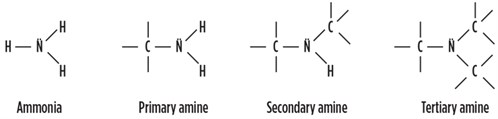
Amines are organic compounds with ammonia (NH3) as the fundamental base compound. By replacing one or more of the hydrogen (H2) atoms with an organic hydrocarbon group, different types of amines are formed—i.e., a primary amine, a secondary amine and a tertiary amine (FIG. 1).
 |
|
FIG. 1. Amine types. |
DEA is generally less reactive than MEA. Applications with appreciable amounts of COS and carbon disulfide (CS2), such as refinery gas streams, can generally be treated successfully. Advantages include resistance to degradation from COS and CS2 and low solvent vapor pressure, which result in potentially lower solvent losses and a reduced corrosive nature when compared to MEA, as well as a lower solvent cost.
Disadvantages of DEA include lower reactivity compared to MEA and DGA—essentially nonselective removal in mixed acid gas systems due to inadequate CO2 slip.7,8 DEA also has higher circulation requirements and cannot be reclaimed by conventional techniques.
MDEA solvents have gained a significant share of the market over the past decade. Their commercial success is due to the ability of MDEA to selectively remove H2S when treating a gas stream containing both H2S and CO2,9 while slipping a significant portion of the CO2. This CO2 slippage can be useful in applications requiring upgrade of H2S content for sulfur plant feed gas or for adjusting the CO2 content of the treated gas while removing H2S to 4 ppm.
Originally, the most significant application of MDEA and formulated MDEA solvents was for tail gas treating units, but the formulated solvents have increasingly displaced primary and secondary amines in refinery treating systems and in high-pressure natural gas applications. MDEA has low vapor pressure, which results in potentially lower solvent losses, less corrosivity, high resistance to degradation, and efficient energy utilization for capital and operating cost savings.
Disadvantages of MDEA include higher solvent cost than MEA, DEA or DGA; lower comparative reactivity; and inability to be reclaimed by conventional techniques.
Batch setup and chemical analysis. The properties of rich acid gas feed to the absorber at the GUPCO plant include:
- Flowrate = 12 MMsft3d
- Temperature = 41°C
- Pressure 49 kg/cm2
- Concentration of H2S feed to contactor = 600 ppm.
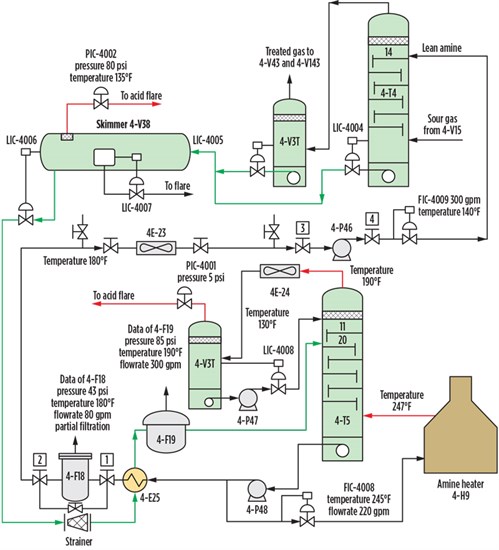
TABLE 1 shows the composition of rich feed gas to the amine unit. FIG. 2 shows the batch setup for the GUPCO amine unit. To evaluate the efficiency of the tray and packed columns and to check the thermodynamic models, DEA and MDEA treatments were modeled in the simulation program.a
 |
 |
|
FIG. 2. Process flow diagram of amine unit at GUPCO plant. |
In the simulation, the acid gas enters the absorber from the bottom, while the amine solution enters from the top. The amine is readied for reuse by passing the stream through a mixer, where it is mixed with makeup water. More of the amine solution may be added to the mixer to ensure the optimal composition for gas sweetening.
A booster pump then raises the pressure to 47 bar, which is the operational pressure of the absorber. A heat exchanger cools the amine solution to the desired operational temperature of 47.3°C. The sweetened gas from the absorber is sent to the pipe system for transport to an onshore system for distribution.
The bottom liquid is then sent to a flash tank, where the stream is heated to flash out most of the hydrocarbons in the stream. The amine solution is then sent to a distillation tower, where the stream is cleaned from H2S to the same level as the sweetened gas. Water is added to the recovered amine and sent to the mixer to be reused for the new sweetening cycle.
The simulation showed H2S removal at 100%, and removal of both H2S and CO2 of 99.07% for the 32 wt% DEA solution. FIG. 3 shows the simulated concentrations of H2S and CO2 mole fractions with the tray number in the top of the absorber. The concentration of H2S in the full rich acid case gradually decreased from Tray No. 24 to zero at Tray No. 15, and the concentration of CO2 gradually decreased from Tray No. 24 to zero at Tray No. 3. Therefore, the removal of H2S and CO2 is 99.07% at Tray No. 1.
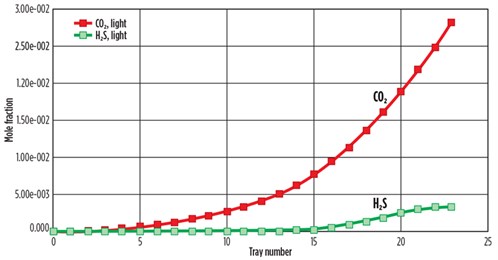 |
| FIG. 3. Composition of feed acid gas with tray position in absorber for DEA treatment. |
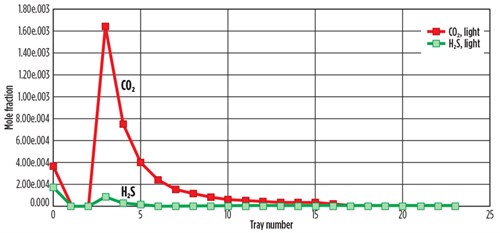
FIG. 4 shows the simulated concentrations of H2S and CO2 mole fractions with the tray number in the top of the desorber. The concentrations of H2S and CO2 at Tray No. 3 in the full rich acid case gradually decreased to zero at Tray No. 15.
 |
|
FIG. 4. Composition of feed acid gas with tray position in desorber for DEA treatment. |
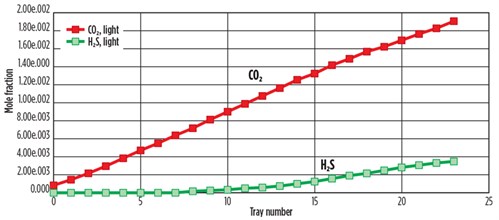
For the 32 wt% MDEA solution, H2S removal was 99.95%, and removal of both H2S and CO2 was 68.85%. FIG. 5 shows the simulated concentrations of H2S and CO2 mole fractions with the tray number in the top of the absorber. The concentrations of H2S and CO2 at Tray No. 24 in the full rich acid case gradually decreased to zero at Tray No. 10. CO2 decreased gradually, but was not completely removed. Removal of H2S and CO2 from sweet gas was 68.85% at Tray No. 1.
 |
|
FIG. 5. Composition of feed acid gas with tray position in absorber for MDEA treatment. |
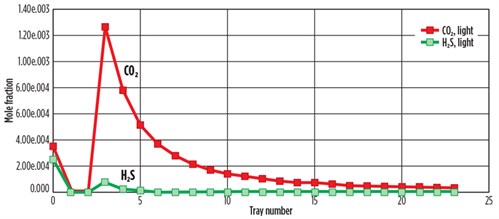
FIG. 6 shows the simulated concentrations of H2S and CO2 mole fractions with the tray number in the top of the desorber. The concentration of H2S at Tray No. 3 in the full rich acid case immediately decreased and reached zero at Tray No. 5. The concentration of CO2 gradually decreased from Tray No. 3 to Tray No. 22, but CO2 was not removed completely.
 |
|
FIG. 6. Composition of feed acid gas with tray position in desorber for MDEA treatment. |
Results and discussion. The simulation results are considered to be reliable, since the thermodynamic model takes temperature, pressure and composition into consideration when evaluating the equilibrium of the reaction.
When comparing the results of the simulated removal processes for the DEA and MDEA solutions, it can be seen that DEA is the less costly and more effective removal solution:
- DEA results:
- Removal of H2S from sweet gas = 100%, and removal of H2S and CO2 = 99.07%
- Average price of DEA chemical = 609,792 LE (Egyptian pounds)/d
- Total mass flow required for DEA = 794 kg/hr.
- MDEA results:
- Removal of H2S from sweet gas = 99.95%, and removal of H2S and CO2 = 68.95%
- Average price of MDEA chemical = 1,207,296 LE (Egyptian pounds)/d
- Total mass flow required for DEA = 786 kg/hr.
The simulation results show that both DEA and MDEA are effective sweetening agents for acid gas. DEA provides the cheapest and most efficient treatment option overall, with H2S removal of 100% and H2S and CO2 removal of 99.07%.
By contrast, H2S removal with MDEA is 99.95%, and removal of both H2S and CO2 is 68.85%. MDEA is both more expensive and more environmentally friendly than DEA, and it removes H2S faster. However, initial CO2 removal with MDEA did not reach acceptable levels, and it was necessary to add more MDEA and water to adequately sweeten the gas. GP
NOTE
a Aspen HSYS
LITERATURE CITED
- Miller, L. N., R. A. Macriss and T. S. Zawacki, “Process for acid gas removal from gaseous mixtures,” US Patent US4080424A, March 21, 1978.
- Kohl, A. L. and R. Nielsen, Gas Purification, Gulf Professional Publishing, Houston, Texas, August 28, 1997.
- “Hydrogen sulfide,” PubChem Public Chemical Database, U.S. National Center for Biotechnology Information, February 8, 2020, online: https://pubchem.ncbi.nlm.nih.gov/compound/Hydrogen-sulfide
- Mahajani, V. V. and P. V. Danckwerts, “The stripping of CO2 from amine-promoted potash solutions at 100°C,” Chemical Engineering Science, Vol. 38, No. 2, 1983.\
- Cornelissen, A. E., “Simulation of absorption of H2S and CO2 into aqueous alkanolamines in tray and packed columns,” Chemical Engineering Research and Design, Vol. 58, No. 4, 1980.
- Weaire, D. and S. Hutzler, “The physics of foams,” Physics Today, Vol. 54, No. 3, March 2001.
- Astarita, G., D. W. Savage, and A. Bisio, Gas Treating with Chemical Solvents, J. Wiley and Sons, Hoboken, New Jersey, 1983.
- Young, E. E., “ A method of preparation of sulfone and its homologues,” Belgium Patent 616856A, October 25, 1962.
- Huntsman Chemical Corp., “Choices of gas treating,” Brochure, 1995.
 |
Nady S. Abbas is Process Section Head at Gulf of Suez Petroleum Co. (GUPCO) in Cairo, Egypt. He has 10 yr of working experience in gas production and processing, with expertise in conceptual design of chemical processes, plant commissioning, startup and troubleshooting. He is also highly experienced in dynamic process simulation and process control. He holds an MSc degree in chemical and petroleum refining engineering and a PG diploma in chemical engineering, both from Minia University in Minia, Egypt. He also holds a BSc degree in petroleum refining engineering from Suez Canal University in Suez, Egypt.
Hassanein M. Asfour is a Professor in the Department of Engineering at Minia University in Minia, Egypt.
Mohammed Z. A. Wahab is a Professor in the Department of Engineering at Minia University in Minia, Egypt.
 |
Eman A. Ashour holds a PhD and is a Professor in the Department of Engineering at Minia University in Minia, Egypt.




Comments