Gas processing technology for new engineers
Readers have demanded editorial content showcasing essential knowledge, common concepts and processes, and continuing education in the gas processing industry—and Gas Processing & LNG has responded. In the first of this teaching series of articles, the author explores the fundamentals of gas processing technology and processes commonly used around the world. Keep an eye out for new “Back to Basics” articles in forthcoming issues of Gas Processing & LNG.
Historically, natural gas has been distributed as a priority in the industrial sector, given the greater territorial concentration of consumption and the consequent lower supply cost. After its distribution for use it is provided to the civil sector; now, as a result of environmental pressures, it is also provided to the transportation sector, in particular the bunkering sector.
Worldwide, the civil and transport sectors absorb more than 56% of global demand. The energy generation sector is close behind in terms of usage and is growing faster than the other two sectors as a result of the increasing electrification of developing world regions. The transport sector absorbs a small fraction of demand, but it is the market with the highest level of growth.
Natural gas is used in many markets, each of which has specific quality requirements that result in different configurations of treatment plants. This article describes the different process architectures in operation in relation to outlet markets for natural gas, along with the individual unitary operations of which they consist.
The natural gas production chain. Natural gas is a complex mixture of chemical species in which methane is the main component. The other constituents are compounds of the homologous series of alkanes (ethane, propane, butane and higher members), aromatics (benzene, toluene, ethylbenzene, xylene and others, generally referred to as BTEX); carbonyl sulfide (COS); carbon disulfide (CS2); mercaptans; and inorganic compounds including water (H2O); hydrogen sulfide (H2S); carbon dioxide (CO2); hydrogen (H2); oxygen (O2); inert gases such as nitrogen, helium and argon (N2, He, Ar); and traces of mercury (Hg).
The bulk of the components with a molecular weight greater than that of methane can be extracted from the mixture as condensates and then sent to a fractionation plant to be recovered individually. Table 1 shows the liquids extracted from natural gas and their uses.
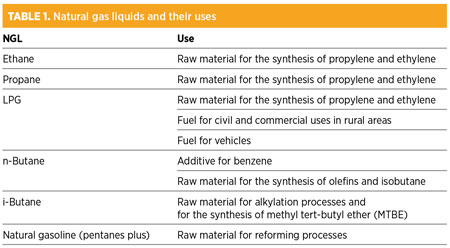 |
The suitability of extracting condensates depends largely on the price difference between oil and natural gas, but in fact the price of condensates tends to follow that of crude oil. Therefore, on an equivalent-energy basis, when the price of crude oil exceeds the price of natural gas, the extraction of condensates from gas is economically worthwhile.
The price difference between crude oil and condensates is defined in the markets as the “frac spread,” while the value of heavies in the gas is indicated as the “shrinkage,” since it represents the loss of value of the natural gas as a result of the reduction of its lower heating value (LHV) after extraction of the liquid. Note: In the markets, the unit price of natural gas is expressed as €/MW ($/MBtu), so if the LHV of the gas is reduced, the same volume of gas entering the pipeline will generate a lower cash flow.
Decisions to invest in NGL are based on the optimal combination of frac spread and shrinkage, provided that an outlet market exists near the plant for condensates, along with satisfactory transport and storage infrastructures.
The midstream of natural gas. The production of natural gas is the first link in the value chain that connects production wells to the end user. The so-called midstream sector is an integral and essential part of this chain. Generally, the midstream refers to the gathering system, conditioning, compression and other processes the gas undergoes before entering the transmission system. Pipeline operators generally impose certain requirements before accepting treated gas into their systems.
Water is a source of corrosion and can lead to the formation of hydrates, which cause erosion. For this reason, natural gas must be treated before entering the transport infrastructures. Its dewpoint must be lower than the transport specifications, based on the ambient temperature. In equatorial climates, it is not unusual for a dewpoint of 0°C–10°C to be required, corresponding to a water content of 4 lb/MMsft3 (minimum)–7 lb/MMsft3 (maximum).
Gasolines (C5+), the heaviest hydrocarbons of pentane, can condense during transport. This condensation can lead to the formation of slugs. These slugs are accelerated by the gas and exert uncontrolled, impulsive forces on the fitting of the pipeline, with possible structural damage. Pipeline operators must impose a maximum dewpoint value for the heavies contained in the gas in transit through their pipelines. In Europe, a maximum dewpoint for C5+ of between –5°C and 0°C is typically specified.
The aforementioned specifications overlap with the so-called sales specifications, which impose limits on the levels of inert gases (CO2, N2). These inert gases can have negative impacts on the LHV and commercial value of the gas, and on sulfur compounds (H2S, mercaptans), due to their harmfulness.
If the natural gas produced by a particular deposit is intended for a small number of users, the processes that the gas undergoes reflect the specific requirements of those operators rather than general transport/sale specifications. Midstream processes can be classified according to two main categories: gas conditioning and the extraction of condensates (NGL).
Gas conditioning. The phrase “gas conditioning” generally refers to the removal of gaseous contaminants, such as H2O (dehydration processes), H2S (sweetening processes), organic compounds of sulfur (CS2, COS, mercaptans), CO2 and Hg. The required level of removal is established by the sales specifications and by the commercial specifications required for NGL.
Dehydration, the process most commonly used in the midstream sector, can be carried out with three different processes:
- Absorption with glycols, in particular triethylene glycol (TEG), when the gas is destined for entry into pipeline systems.
- Absorption on solids when the work cycle entails the extraction and recovery of NGL or for the production of LNG, requiring water dewpoints below –150°C. In these cases, the use of molecular sieves is mandatory; silica gel, on the other hand, has gas dewpointing applications.
- Condensation using refrigeration cycles or by the Joule-Thomson effect; these processes require the addition of inhibitors (either MEG or CH3OH) to the gas to prevent the formation of hydrates. The liquids solvents are recovered, regenerated and reinjected into the supply gas in the closed cycle.
Sweetening is the process whereby H2S is removed from natural gas; CO2, also being acid in nature, is co-absorbed along with sulfuric acid. The processes that can be used to remove these components from natural gas include:
- Washing with chemical solvents, including aqueous solutions of alkanolamine—e.g., monoethanolamine, diethanolamine, methyl-diethanolamine (MDEA), diglycolamine and diisopropanolamine. The primary and secondary amines are not generally selective for the multiple absorption of H2S and organic compounds of sulfur, so there is a growing tendency to use specialist solvents obtained by mixing tertiary amines (MDEA) with other chemicals, both to obtain the desired levels of selectivity and for the simultaneous removal of CO2 and organic/inorganic compounds of sulfur.
- Washing with physical solvents, which do not react with acid gases, but instead perform the desired removal by exploiting the different solubility of gases in different physical solvents. In general, physical solvents are used in applications where the partial pressure of acid gases is high and/or multi-absorption is required. Compared with chemical solvents, physical solvents are less corrosive and require less energy to be regenerated, but they also tend to co-absorb hydrocarbons with a consequent net loss of production. In almost all cases, physical solvents, being proprietary, require a licensing agreement for use.
- Absorption on solid beds by absorption on solid surfaces, as in the case of molecular sieves or by reaction with metal oxide. These processes can be profitably used when the quantities of sulfur to be removed are small (less than 30 kg/Gg–40 kg/Gg).
- Separation with membranes, which are widely used for removing CO2 from natural gas. Membranes in general are not effective for removing H2S and other sulfur species because they cannot guarantee the low concentrations required in the permeate.
The final conditioning process is mercury removal. Hg is a metal that is extremely toxic and harmful to human health; therefore, it is necessary to reduce it to less than a few parts per billion (ppb) in natural gas. By forming amalgams with aluminium (Al), it corrodes the heat exchangers in the cold boxes of the plants carrying out NGL deep extraction or liquefaction for the production of LNG. Processes for removing Hg are mostly based on absorption on absorbent beds, or chemical reactions between sulfur and metal sulfides in which a solid matrix (e.g., activated Al) is impregnated. Note: Hg absorbents cannot normally be regenerated; once saturation has been achieved, the used beds must be appropriately disposed of as toxic or harmful waste.
Fig. 1 shows a process diagram of a typical plant constituting the central processing facility (CPF) for the production of natural gas intended for civil use, for the production of electricity and for regional sale. Note: The quality of the gas produced in the configuration shown in Fig. 1 is compliant with transport and sales requirements. No other treatment is envisaged apart from checking the network pressure, odorization and tax measures in the downstream. However, in the case of petrochemicals, the front end of the H2S content is reduced by 4 MMsft3–7 lb/MMsft3 to fractions of ppm to avoid poisoning the catalysts. In this field, the most widely used system is based on the process of chemisorption on fixed beds.
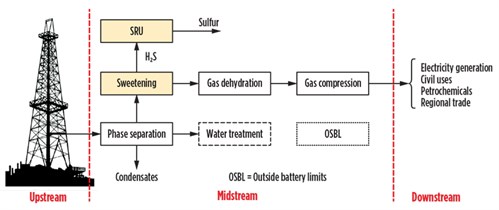 |
| Fig. 1. Block diagram of basic processing facility. |
Sometimes the CPFs are located close to the outlet markets. In regions where infrastructure is lacking, it would not be economically profitable to methanize the area to satisfy the energy demand of rural populations, but it may be appropriate to extract LPG (mixtures of C3 and C4 with small quantities of C2) from the natural gas to supply the rarefied regional market. The plant architecture, in these cases, is configured as shown in Fig. 2.
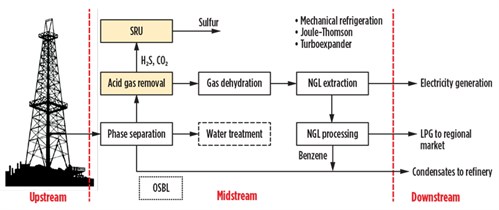 |
| Fig. 2. Block diagram of gas conditioning and LPG/condensates recovery process. |
Extraction and purification of condensates. As discussed, chemical species with a greater molecular weight than methane can be extracted from the gas to maximize the profitability of plants if they are located close to markets where liquid fuels have a higher value than natural gas. The processes whereby NGL can be removed include:
- Mechanical refrigeration, whereby the condensation of heavies is caused by cooling the gas for heat exchange in the evaporators of the appropriate refrigeration cycles. This process is used for controlling the dewpoint of hydrocarbons and is often found as the primary refrigeration phase in the extraction of condensates from natural gas and in combination with other refrigeration options. The most commonly used coolant is propane; ammonia or other cooling fluids are used in some applications. In general, the minimum achievable operating temperature is determined by the normal boiling temperature of the coolant selected: typically, it ranges from –34°C to –40°C for the main commercial coolants.
- Lamination, whereby the condensation of liquids is induced by cooling of the gas, caused by isenthalpic expansion of the gas (known as the Joule-Thomson effect). Note: In industry, this process also goes by the name of low-temperature separation (LTS). While this process has the virtue of simplicity and, therefore, reduced CAPEX, the pressure drop required by the expansion (sometimes 30 bar–60 bar) is disadvantageous in terms of OPEX. LTS is typically used in hydrocarbon dewpointing units.
- Turboexpansion, whereby the condensation of liquids is induced by cooling of the gas, which is caused by isenthalpic expansion of the gas, using rotating machines. Since the expansion produces work, the final temperature of the gas is lower than can be obtained with the Joule-Thomson effect or with simple mechanical cooling (assuming the same pressure jump). The main application of this process is the boosted extraction of NGL, whereby the gas can be cooled to less than 173 K. Sometimes it is also used in a hydrocarbon dewpointing context instead of Joule-Thomson. This is because, at the same final temperature, a smaller pressure drop occurs, with a consequent reduction of recompression costs.
- Silica gel, which has a certain affinity with C5+ in addition to water. This affinity is exploited to simultaneously remove water and heavies from natural gas, as appropriate. The best-known applications include the conditioning of natural gas extracted from stocks in exhausted oil deposits and gas conditioning at high pressure, for which the thermodynamic behavior of gaseous mixtures renders mechanical refrigeration ineffective.
- Other processes. Before 1970, the most commonly used system for extracting NGL from natural gas was based on the process of absorption on a solvent (benzenes or naphtha) at a low temperature. This technique has been almost entirely replaced by turboexpansion. As mentioned, natural gas also contains appreciable quantities of inert gases, in particular N2 and He. The former, if contained in significant quantities, lowers the gas’ LHV, while the latter, being a commercial gas, can be extracted as appropriate from the mixture for entry into the technical gas market. This operation is carried out by modifying, as appropriate, the cryogenic distillation section of the plant’s NGL extraction unit. If it is necessary to remove small quantities of N2, however, pressure swing absorption (PSA) units should be installed upstream of the condensate extraction section, rather than modification of the cold box.
The condensates extracted from the gas contain significant quantities of methane and light ends (low-molecular-weight hydrocarbons). These light components must be removed in the stabilization section, positioned downstream of the extraction, before the liquid produced can be sold. Stabilization assumes different lineups depending on the application and the desired product.
For hydrocarbon dewpoint applications, or to recover only C5+ from an associated gas, the stabilization level is given by the Reid specification for vapor tension admissible for the commercial condensate, similar to what occurs in the stabilization of crude currents.
In processes where extraction is boosted to the maximum degree, the condensate consists of C2+ and the only light to be removed is methane. This operation is carried out in a distillation column called a demethanizer. If the economics of the plant suggest recovery of LPG only, then the operating parameters of the stabilizer are set to obtain mainly ethane in the overhead; this type of stabilizer is called a deethanizer.
Finally, the stabilized condensates undergo purification treatment to bring the quality of the liquids in line with commercial requirements. The purification treatments group together two categories of processes:
- Removal of CO2 from the C2+ currents. The natural gas supplied to a plant contains CO2, which tends to be concentrated in the condensates rather than in the gas. To satisfy the sales specifications (CO2 concentration < 500 ppmw), the liquid must undergo a CO2 removal process, typically a liquid-liquid extraction based on amines.
- Removal of sulfur and its compounds. H2S and its organic compounds tend to be concentrated in the liquid fractions produced from natural gas. To satisfy sales specifications for condensates, in particular the copper test requirement, the liquid currents must be treated with appropriate purification processes based on molecular sieves, caustic washing, amines, etc.
In view of these points, a plant architecture that carries out the extraction of condensates from natural gas, with integrated liquefaction of natural gas, is shown in Fig. 3. A summary of the alternative processes used for the various production phases is included.
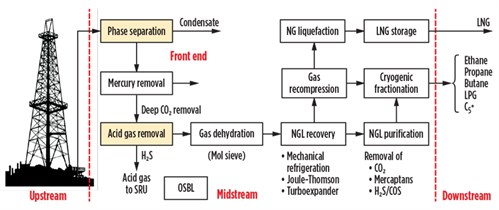 |
| Fig. 3. Block diagram of an integrated NGL and LNG liquefaction process. |
Growing trends in gas processing. A new and fast-growing trend that is an alternative to reinjection, particularly in the case of associated gas in an offshore environment or for gas reserves close to low-demand markets, is compressed natural gas (CNG). In CNG, the natural gas is stored at high pressure (200 barg–250 barg) in special storage that maximize the natural gas/module mass ratio.
Another area of development is gas-to-wire, whereby electricity is produced at the well opening to satisfy local demand. In turn, gas-to-wire is evolving towards gas-by-wire, whereby more distant users are reached through the adoption of new transmission technologies for high-voltage, direct-current electricity. The technological development of systems for alternate-current/direct-current conversion (and vice versa) is based on this concept. GP
Lorenzo Micucci is a Senior Director at Siirtec Nigi SpA. He has more than 30 yr of experience in the engineering and contracting industry, most of which has been spent in the natural gas sector. In 2001, he joined Siirtec Nigi in Milan, where he directed the process design and operations department and the research and development department. During his time as R&D head, three patents have been granted to Siirtec Nigi, two of which have been implemented on an industrial scale. At present, he is the Senior Director of the technology and marketing departments. Mr. Micucci also worked for Saipem (Snamprogetti) as a Plant Designer for integrated gasification combined cycle and gas-to-liquids plants. He holds an MS degree in chemical engineering from the University of Bologna in Italy and is enrolled as a Qualified Engineer in the Register of Milan Order of Engineers.




Comments