Choose the correct heat transfer fluid for gas processing operations
J. Seybold and A. Davis, Radco Industries, Batavia, Illinois
In recent years, the gas processing industry has seen rapid growth worldwide. The US, Russia, Iran and Qatar are thought to have the largest natural gas reserves in the world. In the US, Canada and China, shale gas production is increasing. Shale gas potential is also being studied in parts of South America and Africa. The growth of the natural gas market has created a high demand for gas processing plants and major pipelines in natural gas-rich areas of the world.
Raw natural gas has impurities that must be removed before being processed further. These impurities typically include water, carbon dioxide, hydrogen sulfide, methane, ethane, butane and the BTEX compounds (i.e., benzene, toluene, ethylbenzene and xylene), which are volatile organic compounds. These impurities are removed in a series of processes, some of which use heat transfer (HT) fluids. Choosing the correct HT fluid is based on a number of different factors and requires technical support from an HT fluid expert.
Choosing a heat transfer fluid. The most important considerations when choosing an HT fluid are the fluid’s key operating temperatures. The startup temperature should be defined at the pumpability point, which is the lowest temperature at which a pump can handle the viscosity of an HT fluid at low temperatures. The pumpability point is the temperature at which the fluid’s viscosity is about 300 centistokes (cSt). The desired maximum bulk fluid operating temperature is important to determine which type of HT fluid is used. The flash point and autoignition temperature are used to establish safety guidelines and procedures.
The pumpability point is especially important in cooler climates, such as Russia, Canada and the US Midwest. A growing demand exists for gas processing in these regions of the world, where temperatures sometimes reach –49°F (–45°C) during the winter. At low temperatures, some HT fluids have very high viscosities, making shipping and startup difficult. This is where the pumpability point of the HT fluid becomes important. The lowest temperature at which the system pumps can handle HT fluids is the temperature at which the viscosity of the fluid is approximately 300 cSt—i.e., the pumpability point.
The standard HT fluids typically used in gas processing applications are mineral oil based. Synthetic-based HT fluids are also used, depending on the desired maximum bulk operating temperature. Mineral oil HT fluids are generally operated up to 600°F (316°C), while synthetic HT fluids can be operated above 600°F (316°C) and, in some cases, up to 750°F (399°C). Both mineral oil and synthetic HT fluids are favored in gas processing plants due to their high flashpoints, high autoignition temperatures and low vapor pressures.
Diesel and kerosene are occasionally used in petrochemical processes, but they have several disadvantages that make them more difficult to use. Both diesel and kerosene have low flashpoints and autoignition points, making them combustible fluids that require additional safety precautions. They also have much higher vapor pressures at the higher operating temperatures than the mineral oil and synthetic HT fluids. This means that they have a higher volatility and, therefore, vaporize quickly, requiring more frequent replacement of fluid in the system.
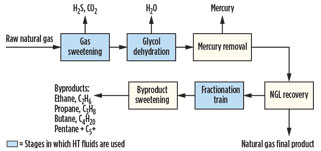
Heat transfer fluids in gas processing. As shown in Fig. 1, raw natural gas goes through a series of processes to remove impurities before the final product is ready for consumption. The raw gas is rid of hydrogen sulfide (H2S), carbon dioxide (CO2), water (H2O) and mercury through the gas sweetening, glycol dehydration and mercury removal stages. Not included in
Fig. 1 is the nitrogen (N) rejection stage, where N is removed from the gas. This stage is uncommon and does not typically use HT fluids. The gas is then sent to NGL recovery, where the pure, final product gas is removed for sale to end users.
 |
|
Fig. 1. Gas processing flow diagram. |
The recovered NGL is sent to the fractionation train and sweetening units to purify the byproducts: ethane (C2H4), propane (C3H8), butane (C4H10) and a mixture of pentanes and heaver hydrocarbons (C5+). HT fluids are used in the stages highlighted in blue in Fig. 1: gas sweetening, glycol dehydration and the fractionation train.
Gas sweetening. The gas sweetening process, also known as acid removal or amine gas treating, is the first important stage of gas processing in which the acid gases, CO2 and H2S, are removed from the raw gas. Being acid gases, they can cause corrosion in certain systems. The raw gas—referred to as sour gas due to its odor—is crossed with an amine gas in an adsorber, thereby removing the acid gas. The product stream is referred to as sweetened gas, as the removal of CO2 and H2S greatly improves its scent. The amine and acid gas mixture enters the regenerator, where it is stripped of the acid gases and recycled back into the absorber.
The regenerator is heated with a reboiler, which, in turn, is heated with an HT medium. Traditionally, steam has been the HT fluid of choice. However, because steam causes corrosion, it requires anti-corrosion additives. Many times, additional maintenance personnel are needed to tend to the system. Due to these extra precautions, many gas plants have begun using synthetic or mineral oil HT fluids in place of steam.
Glycol dehydration. After the acid gas is removed, the product gas stream enters the glycol dehydration stage, in which moisture is removed. Moisture in the product gas stream, if not removed, has the potential to freeze downstream or form compounds called hydrates with CO2 and hydrocarbons, both of which can plug piping and system equipment. Excess moisture can also cause corrosion.
Like the gas sweetening stage, glycol dehydration consists of an absorber and a regenerator. Glycol is first crossed with the gas stream to absorb any moisture. The moisture-free gas, or dry gas, is then moved to the next stage of the process. The wet glycol is fed to a regenerator where the moisture is evaporated, and the regenerated glycol is sent back to the absorber. Also, as in the gas sweetening stage, the regenerator in the glycol dehydration stage is heated by a reboiler, which is typically heated by a synthetic or mineral oil HT fluid.
Fractionation train. After the product gas stream is ready for consumption, the LNG stream that remains can be processed further into byproducts to be sold or used as feedstock in other areas of the plant. This is either done in the same plant or at a separate fractionation plant. A series of three distillation columns remove ethane, propane and butane from the stream in succession. The bottoms of the final distillation column contain pentane and other heavy hydrocarbons. Each of the three distillation columns has a reboiler that is heated with synthetic or mineral oil HT fluid, often at temperatures above 500°F (260°C).
Bath heaters. Indirect bath heaters (Fig. 2) are used in many different areas of gas processing to heat gas and process fluids, and to prevent the cooling effects of expanding gases. They are normally designed to use a glycol/water mixture as the HT medium at temperatures up to 300°F (149°C). Recently, bath heaters have been required to reach temperatures outside of the range of glycol-based HT fluids, closer to 400°F and higher. When glycols are used at these temperatures, they degrade, become acidic and can cause corrosion in the system. For this reason, there has been a shift to mineral oil and synthetic HT fluids, as they are capable of higher temperature ranges.
 |
|
Fig. 2. Two 25-MMBtu/hr downfired hot oil heaters, with |
A system that is being converted from glycol-based HT fluid to a mineral oil or synthetic HT fluid should be thoroughly cleaned to ensure the complete removal of the glycol/water mixture. This is important because residual glycol and water will degrade the mineral oil or synthetic HT fluids over time. As exposure to air can also cause oxidation and degradation in mineral oil and synthetic HT fluids, a blanket of N should be added to the system. A circulation pump should also be added, if possible, to prevent the fluids from exceeding their maximum film temperature and degrading.
Annual fluid analysis program. The most important part of using HT fluids in any system is testing it regularly and keeping it well maintained. This can and should be done with the help of your HT fluid supplier by establishing a regular fluid analysis schedule. HT fluid that is not maintained properly will degrade and impact the system’s operation.
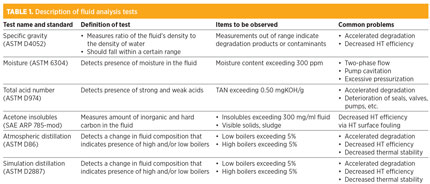
The standard tests that should be done include specific gravity (ASTM D4052), moisture (ASTM 6304), total acid number (ASTM D974), acetone insolubles (SAE ARP 785-mod) and atmospheric and simulated distillation (ASTM D86 and ASTM D2887, respectively) to determine high and/or low boilers (fluids that have higher or lower boiling points than the HT fluid itself). A description of each ASTM test, and the common problems encountered during them, are described in Table 1.
 |
Takeaway. As detailed here, HT fluids are a critical component of gas processing. It is important to understand which type of HT fluid best fits not only the application, but also the geographical region of a gas processing plant.
It is best to work closely with an HT fluid supplier to ensure the proper selection of an HT fluid. Fluid analysis helps lengthen the life of HT fluid and minimize performance issues. GP
 |
Jed Seybold is responsible for the growth of Radco Industries’ heat transfer (HT) fluids product division. He has extensive knowledge of the HT fluids industry, with over 11 years of experience in sales and technical support. He has worked in a number of capacities that include domestic and global sales, sourcing and product development of HT fluids. Mr. Seybold also has more than 36 years of chemical sales and business development experience. His achievements include a US patent for PQ Corp. Prior to joining Radco Industries, he served as senior global business development manager for Paratherm, a subsidiary of Lubrizol Corp. Mr. Seybold earned a BS degree in chemical engineering and a BA degree in chemistry from Louisiana Tech University in Ruston, Louisiana.
 |
Alison Davis is a recent graduate of the University of Michigan College of Engineering in Ann Arbor, Michigan, where she earned a BSE degree in chemical engineering with an international minor. She has joined the product development and testing team at Radco Industries, and will contribute to a number of planned product introductions in the near future. Prior to joining Radco Industries, Ms. Davis served an internship at Flexfab LLC. Her experiences included collecting and analyzing prototype tests, participating in product return analysis of failed products and programming testing equipment to meet testing specifications. Ms. Davis is also a member of the American Institute of Chemical Engineers (AIChE).




Comments