Reduce gas plant costs and shutdowns with appropriate antifoam substitutes
N. A. Al-Qahtani and N. K. Al-Subaei, Saudi Aramco, Haradh, Saudi Arabia
A frequent problem in gas plants is foaming, which occurs during the gas sweetening process. This problem is usually the result of contaminants entering the process and improper operating conditions. The first mitigation action is normally the addition of a chemical antifoaming agent. The second is to find the cause and eliminate it. However, antifoaming agents can be an effective interim—or even long-term—cure if elimination of the cause is not possible.
Antifoaming agents have a wide range of applications in industry. Many formulations are not suitable for gas treating uses and can actually aggravate the situation. Also, antifoaming agents are usually non-ionic surfactants that act to lower the surface tension of the vapor/liquid interface, reducing liquid film strength and surface viscosity and speeding drainage from bubbles. These chemicals are intended to facilitate gas and liquid disengagement by weakening the cell structure of the bubbles. Antifoaming agents have no positive contaminant-removal properties. The injection of these chemicals into recirculating amine solutions is common, and even considered necessary for normal plant operations.
Based on extensive experience in facility operations and commercial applications, a high-performance antifoam agent specifically designed for gas sweetening uses is considered. The B-5A antifoaming agent, one of the solutions considered in this study, provides foam control in the amine sweetening process.
Process overview. Gas sweetening and acid gas removal at the Haradh gas plant (HGP) in Saudi Arabia use an aqueous solution of alkylamines (methyldiethanolamine solvent, or MDEA) to remove hydrogen sulfide (H2S) and carbon dioxide (CO2) from sour gas.
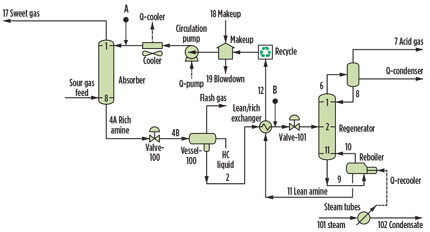
The gas processing unit consists of four gas trains. Two trains are used to treat sour gas feed with a capacity of 385 MMscfd at each train, and the other two trains treat sweet gas with a capacity of 480 MMscfd at each train. The flow diagram of the process unit is shown in Fig. 1.
 |
|
Fig. 1. Simplified amine gas sweetening process flow diagram. Points A and B |
The antifoam injection system at the HGP consists of a chemical storage vessel with dosing pumps linked to control logic that was designed based on the acid gas content in the sour gas feed. Two injection points are used in each sour gas train located in the amine system upstream of the amine absorber and stripper. It is a common practice in gas processing operations to inject antifoam on a continuous basis when it should not be injected, since it is a short-term solution utilized during process upsets.
Methodology. The evaluation was executed in accordance with the following steps:
- Prepare evaluation criteria. A complete evaluation criteria set was prepared that included the lab test criteria developed for foaming amine solutions to identify the appropriate product from board antifoam products. Each product was to be tested and evaluated against the engineering standard criteria and amine manufacturer design.
- Develop a list of potentially qualified antifoam manufacturers. The evaluation team conducted an assessment study on five different antifoaming agents, which are based on silicone, polyglycol and fatty alcohol.
- Prepare an analysis of each product. Detailed analyses of foaming tendency, foam height and foaming stability index “break time” were conducted, in addition to thermal stability tests at operating temperatures.
- Make recommendations. A suitable antifoam (in terms of destroying the foam level with less chemical consumption) was recommended for use, in addition to the dosage rate and the proper setup for the antifoam dosage and storage system.
Technical evaluation. Two parameters can be assessed on the effectiveness of antifoam:
- Knockdown ability and foaming stabilizing index test: the speed to destroy existing foam, and the efficiency of the antifoam over time
- Thermal stability test: the ability to maintain conditions at operating temperatures over time (Fig. 2).
 |
|
Fig. 2. Thermal stability test setup. |
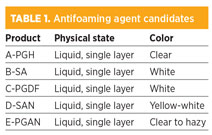
A total of five different antifoam products were sampled in 2-liter (l) batches and used prior to an efficiency approval test, as listed in Table 1.
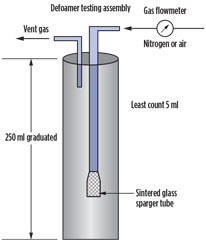
In the experimental procedure, the knockdown ability and foaming stabilizing index test included a bubbling method (Fig. 3). A graduated column with a glass frit in the bottom was used. Foaming MDEA was poured into the column, and nitrogen at a constant flowrate was introduced through the glass frit in the column. Foam was generated by the nitrogen and started to travel up the column. At a determined level of foam, the antifoam was injected. By this method, several antifoaming agents were tested.
 |
|
Fig. 3. Simplified defoamer testing |
Next, the thermal stability test was conducted after the agent with the knockdown ability, and the foaming stabilizing index test was identified. Pure antifoam samples were placed in an oven under storage and operating temperature settings, which are 77°F and 140°F, respectively.
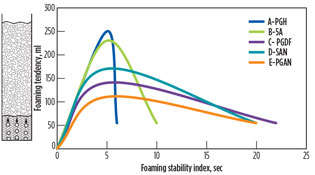
Analysis and results. Five main types of antifoaming agents are used to assess antifoam effectiveness and durability with different material bases. These five agents are classified for use in amine systems. The best agent for use in a specific amine application can be obtained from the foam height vs. the break time. The selected antifoam is the product with the best compromise between shock effect and durability.
 |
|
Fig. 4. Foaming tendency and foaming stability index test |
The best antifoam is A-PGH, since it has the ability to destroy foam levels from 250 ml to 50 ml within 1 sec, and the B-5A agent was able to reduce foam levels from 230 ml to 50 ml within 5 sec. Other agents required more time (approximately 15 sec to 17 sec) to destroy foam levels of 140 ml and 170 ml, as shown in Table 2 and Fig. 4. It was discovered that testing antifoam agents at two different temperatures—those used for storage (Table 2) and operating (Table 3)—did not make a difference in the quality of the destruction of foam levels and or in the break time requirement, as shown in Tables 2 and 3. The total amount of antifoam used to reach the normal level of 50 ml is approximately 0.0993 g.
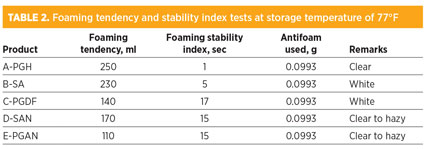
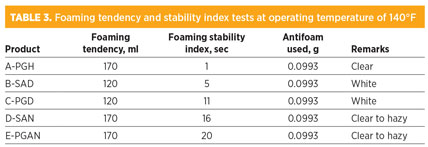
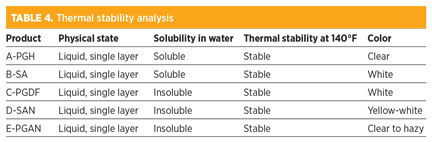
In Table 4 and Fig. 4, all tested antifoam agents showed high stability for products without any indication of decomposition under two different temperatures representing the storage temperature and process operating temperature. Furthermore, A-PGH was discovered to be soluble in water compared with the other agents.
Economic evaluation. Efforts to improve the performance and optimize the dosing rate of antifoaming agents are still continuing. A polyoxypropylene glycol base prepared with an antifoaming agent is said to be highly effective for the prevention of foaming problems in the amine process. Likewise, antifoaming agent A-PGH and B-5A are claimed to be highly effective for the prevention of foaming problems in MDEA solutions.
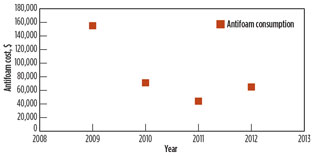
Antifoaming agents promise efficiency over a wide range of temperatures at low dosage levels, stable dispersion with MDEA at typical dosing solution concentrations, and economic viability. However, the use of some agents led to contamination in the amine system and increases in operating costs. The study examined the use of antifoam chemicals totaling 15 Mgal in volume, which corresponded to $65 M/yr for acid gas removal and acid gas enrichment, as shown in Fig. 5.
 |
|
Fig. 5. Antifoam agent consumption for acid gas removal |
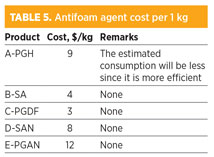
The cost of each chemical is reported in Table 5. The best agent was found to have costs close to the agent presently being used at the HGP. This agent is used in continuous dosage at four injection points, with a total flowrate of approximately 2 gph as pure agent. The proposed chemical can be used as needed, and its dosage will be controlled by amine surface tension technology.
Findings. Study results show the effectiveness of various antifoaming agents:
- The results of foaming tests for antifoaming agent A-PGH reveal that, at a concentration of 15 ppm to 25 ppm, the antifoam solution remains homogeneous, with a foam level of less than 5%.
- Antifoaming agent D-PGAN is insoluble in water and is intended to be injected in its pure form.
- Antifoaming agent D-PGAN is usually mixed with water, resulting in emulation formation, which reduces the amine strength and stable dispersion with MDEA; it also results in pump integrity failure.
- Antifoaming agent A-PGH is fully dispersible in amine solution at the solution concentrations of 15 ppm and 25 ppm, as normally practiced.
- At dose rates of 0.099 gpm by weight, these antifoaming agents were effective at operating temperatures of 70°F to 140°F; moreover, the overall performances of monitored antifoaming agents were found to be satisfactory during these trial periods.
- Use of a surface tension injection technology (STISK) for antifoaming agent A-PGH or B-5A is an appropriate methodology for controlling the dosage of this agent.
- Mixing antifoaming agent C-PGDF and D-5AN with water will result in emulation formation, which reduces the amine concentration strength and stable dispersion with MDEA/DGA solutions, as well as chemical pump failure.
- Antifoaming agent A-PGH is claimed to be highly effective in the prevention of foaming problems in MDEA solution
- Antifoaming agent A-PGH has promising features, such as efficiency over a wide range of temperatures at low dosage levels, stable dispersion with MDEA/DGA solutions at typical dosing solution concentrations, and economic attractiveness.
- The STISK dosing technology is one way to control A-PGH and B-5A dosage, rather than using manual injection or other conventional practices for controlling antifoaming agent dosage.
Recommendations. In an effort to reduce plant operating cost and frequent shutdowns, an overall analysis is performed on the antifoaming agents being used in acid gas removal and enrichment processes to determine the appropriate chemical and dosage mechanism. The existing antifoaming agent and its system performance are evaluated and optimized to establish best practice for the gas sweetening process. A detailed assessment of foaming tendency vs. antifoaming agents investment is carried out to determine potential plant profits. A maximum reduction of 85% in foaming upset in gas sweetening processes can be achieved with the appropriate type of agent and technology for controlling foaming through antifoaming injection to amine loops. This type of antifoaming agent (formulated) can be applied to other gas sweetening process using MDEA and DGA solvents. GP
ACKNOWLEDGMENTS
The authors thank Dr. Bader Mughadi and Mark Thomes, of the gas sweetening process and technology division of the Gas Sweetening Specialist company, for their technical assistance.
 |
Nasser Al-Qahtani is a senior process engineer with more than eight years of experience in the oil and gas industry, specifically in gas sweetening processes. His experience covers conceptual to detailed design and optimization studies on gas sweetening, sulfur recovery and sour water stripper processes. He has developed technical businesses for gas sweetening and sour water stripper treatment processes. Mr. Al-Qahtani is the author of a number of technical and scientific papers. He holds a BS degree in chemical engineering and an MS degree in petrochemical industry, and is lean Six Sigma certified.
 |
Nawaf K. Al-Subaei is an analytical technician with 10 years of industrial experience. He specializes in chromatographic sciences and mass spectrometry. His work includes compositional and trace analyses by single and multidimensional chromatographic separations, as well as electrophoresis with various universal and selective detection, including GC, GCxGC, HPLC/UPLC and CE-MS. Mr. Al-Subaei’s research areas include water, petroleum, petrochemicals (glycols), oil field chemicals (acidizing fluids and additives) and industrial water. He recently worked on a gas sweetening project sponsored by King Abdul-Aziz City for Science and Technology (KACST) and the Atomic Energy Research Institute.




Comments