GTL innovation produces clean base oils from natural gas
S. Gullapalli and A. Falender, Shell Global Solutions, Houston, Texas
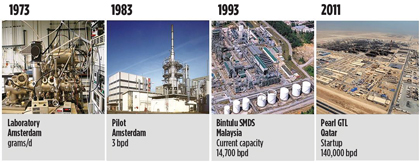
The idea to produce base oil for lubricant applications using natural gas as the hydrocarbon source was developed 40 years ago at the Shell Technology Center in Amsterdam, The Netherlands. Several years later, the reaction was optimized in a laboratory bench-scale reactor. During the 1980s and 1990s, this reaction was scaled up to a pilot plant and then to a small commercial plant.
During this development process, several optimizations were made and captured in more than 3,500 patents. This innovation culminated into a commercial reality with the opening of the world-scale Pearl GTL plant in Qatar in 2011 (Fig. 1). Pearl produces 140 Mbpd of synthetic GTL products and 120 Mbpd of NGL and ethane.
 |
|
Fig. 1. Technology development timeline for Shell’s gas-based lubricants. |
Shell’s GTL process is an integrated process that sequentially converts natural gas to syngas, then to hydrocarbons, and finally to GTL products.1 PurePlus Technology is the Shell-trademarked name in the US for the proprietary GTL process. An important product of this process is a high-quality base oil. Shell is the world’s largest producer of GTL base oils at a commercial scale.
Here, the steps involved in the process of converting natural gas to GTL products are described, with a focus on GTL base oils and fully formulated motor oils.
GTL technology
The heart of Shell’s GTL technology is Fischer-Tropsch (FT) synthesis, based on the catalytic production of paraffin hydrocarbons from carbon monoxide (CO) and hydrogen (H2).2 Natural gas, the cleanest-burning fossil fuel, was chosen as the preferred hydrocarbon source because it is affordable, available and environmentally acceptable.
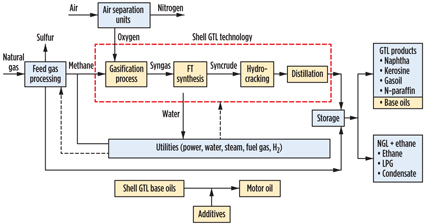
In addition to the FT reaction, there are several other steps involved in the conversion of natural gas to finished base oils and motor oils, as shown in Fig. 2.
 |
|
Fig. 2. Flow diagram of Shell’s GTL process. |
Feed gas processing. To prevent catalyst poisoning in subsequent steps, it is imperative for the gas to be free of contaminants, especially sulfur compounds. In addition, the separation of valuable NGL from the raw feedstock is important. At the end of the feed gas processing step, greater than 99% of sulfur and LPG is recovered.
Desulfurization. There are three steps in the desulfurization of natural gas:
- Bulk removal of hydrogen sulfide (H2S), using an amine treatment step. This stage also functions to remove carbon dioxide (CO2) from natural gas.
- Conversion of H2S to elemental sulfur, followed by offgas treating to further reduce sulfur in flue gas.
- Remaining sulfur is brought to 10 ppbv levels by adsorption.1
NGL. In this assembly, NGL such as LPG and condensate are separated. NGL ethane, propane and butane can be further extracted from LPG.
Trace component removal. Organic chlorine and mercury contaminants in natural gas are removed by adsorbents.
Air separation unit. Cryogenic separation of air is used to obtain oxygen with a purity of approximately 99.8%.
Gasification process. The gasification process refers to the production of syngas and its subsequent purification.
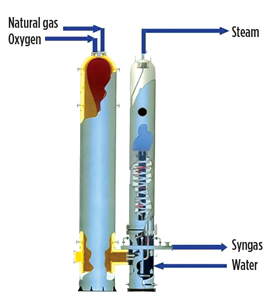
Syngas generation. While there are several syngas generation processes,1 non-catalytic partial oxidation (Fig. 3) is discussed here. Syngas is primarily a mixture of CO and H2. In partial oxidation, the oxidant (oxygen from the ASU or water) and the hydrocarbon (sweet or desulfurized natural gas) are allowed to react, without any catalyst, in a refractory clad pressure reactor with top burners at temperatures of 1,250°C–1,400°C. High-pressure steam, resulting from the raw syngas quench, can be used to run other assemblies.
 |
|
Fig. 3. Non-catalytic partial oxidation syngas generation. |
This process is preferred since it does not require catalysts or steam and is highly tolerant of sulfur, thereby favoring efficiency. Additionally, in partial oxidation, the remaining sulfur impurities in syngas are converted primarily to H2S, eliminating the need for a hydrogenation step in the subsequent purification step.
Purification of syngas. For the FT synthesis, the syngas feed should have very low levels of compounds like H2S, carbonyl sulfide (COS), ammonia (NH3) and hydrogen cyanide (HCN). Purification of raw syngas is an important step, and it is achieved through a combination of adsorption and scrubbing.
Production of hydrogen. H2 production is done through steam methane reforming (SMR) of natural gas, an ex-situ water-gas shift reaction (WGS) and pressure-swing adsorption (PSA).
FT synthesis. The FT reaction is highly exothermic in nature (approximately 165 kJ/mol–180 kJ/mol of CO converted), and it needs to be operated in a narrow temperature range. Reactor design is extremely important to facilitate efficient heat removal and to prevent catalyst deactivation, decreased selectivity of desired final products or uncontrollable runaway reactions. In addition to heat removal, handling the reaction products, such as water, is also necessary to mitigate catalyst deactivation.
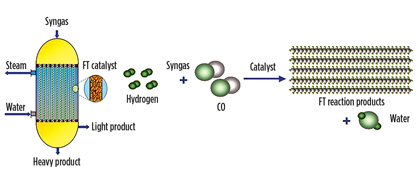
Reactor design. Shell’s GTL process utilizes a multitubular fixed-bed reactor configuration (Fig. 4). Other reactor designs for FT synthesis are fluidized- and ebulated-bed reactors, slurry-bubble column reactors and structured reactors.1 The multitublar fixed-bed reaction has several advantages to the other reactor types, such as higher C5+ selectivity, lower CO2 selectivity, easy scale-up, good isothermal operation, no catalyst attrition, no intra-particle diffusion limitations and no separation of catalyst.
 |
|
Fig. 4. Multitubular fixed-bed reactor (left) and the FT reaction (right). |
Reaction regime. The Shell GTL process runs the low-temperature FT (LTFT, 200°C–260°C) reaction as opposed to the high-temperature FT (HTFT, 300°C–350°C) reaction, even though HTFT consumes a lower H2/CO ratio. LTFT gives preferred products, such as high-quality gasoil and base oils, whereas HTFT products are mainly naphtha and gasoline.1, 3
Catalyst. The GTL process uses an oxide-supported cobalt (Co) catalyst.1 Co was chosen over an iron (Fe) catalyst because it shows greater activity under LTFT operation, higher selectivity toward long-chain hydrocarbons, lower coke formation, lower olefin and oxygenate selectivity, lower CO2 production and higher resistance to deactivation by water.
The use of high surface area and porous metal oxide supports or carriers, in combination with optimized catalyst size and shape, reduces diffusion-limited reactions and optimizes product selectivity. The supports also provide hydrothermal stability to the catalyst. Note: While the support and promoters play an important role, they do not alter the turnover number of the reaction for Co crystallite sizes of greater than 10 nm.
Following the FT synthesis, the light detergent fraction or n-paraffin fraction (C10–C13) is extracted from the FT product and subsequently hydrogenated. This is a premium feedstock for detergent production.
Hydrocracking. The syncrude is then subjected to a hydrocracking step, primarily to isomerize hydrocarbons to improve their cold flow properties. Other reactions include hydrogenation of olefins and oxygenates. GTL hydrocracking is different from refinery hydrocracking because sulfur- and nitrogen-containing species and (poly)aromatics are absent.
In terms of reactor design, however, GTL and refinery hydrocrackers are similar. Typically, a multi-bed trickle-flow adiabatic reactor is used, wherein the hydrogenation of olefins is the fastest reaction, followed by hydrogenation of oxygenates and isomerization of hydrocarbons. Bifunctional catalysts with a strong hydrogenation/dehydrogenation function and medium acid-function catalysts are used in the GTL hydrocracking process.
Distillation. The products of the hydrocracking step are then subjected to atmospheric distillation. Upon distillation, the boiling fractions obtained are LPG, naphtha (30°C–175°C), kerosine (175°C–250°C) and gasoil (250°C–370°C). Although not the focus of this article, GTL gasoils are another important GTL product, since they have extremely high cetane numbers (70–75) as a result of their highly isoparaffinic nature.
Vacuum distillation is applied to the long residue (> 370°C) fraction to obtain the raffinate fraction (370°C–540°C) that is sent to the base oil plant, and a
> 540°C fraction that is recycled to the hydrocracker. The base oil plant consists of a unit that isomerizes n-paraffins and a redistillation unit that converts the raffinate to high-quality base oils.
Finished motor oil. Primarily isoparaffinic in nature and up to 99.5% free of sulfur and nitrogen impurities, Shell’s GTL base oils have beneficial properties such as high viscosity indices, good oxidation stability, low volatility and good cold flow properties. The base oils are then blended with high-performing additives, such as active cleansers, to produce finished motor oils.
Base oils determine the fundamental properties of the lubricant, and the additives help enhance the base oil properties and protect the mechanical parts of the engine. Motor oil formulations consist of approximately 75%–90% base oil and 10%–25% additives. Therefore, the choice of the correct base oil has an important impact on the performance of the finished motor oil.
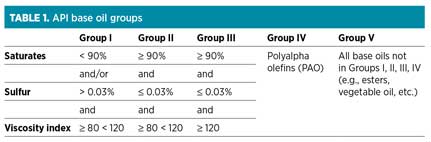
The American Petroleum Institute (API) classifies base oils in different groups based on the minimum specifications shown in Table 1.
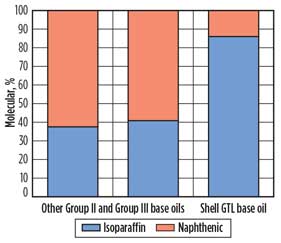
Shell GTL base oils are classified as Group III base oils, but they exceed minimum API specifications. Compared to Shell GTL base oils, other Group III base oils are made by refining and chemically treating crude oil. Fig. 5 shows the difference in composition of Shell GTL base oils to other Group II and Group III base oils.
 |
|
Fig. 5. Composition of different API Group II and Group III |
Shell GTL base oils contain a much greater amount of isoparaffins and a much lower amount of naphthenes than other API Group II or Group III base oils (Fig. 5). The greater isoparaffin content in Shell base oils permits high viscosity indices, good low temperature properties and improved oxidation resistance compared to other Group II and Group III base oils.
Motor oil properties
Motor oils are finished lubricants that are a combination of the base oil and high-performance additives. Several key properties of lubricants for motor oils with different base oils were tested.
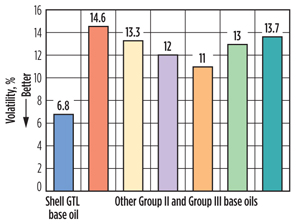
Volatility. Volatility characteristics of different motor oils of SAE 5W-20 grade were tested using the ASTM D5800 test procedure. Shell GTL base oils enable motor oil formulations to have NOACK volatilities of 10% and lower. Typically, Group II and other Group III base oils cannot achieve such low volatilities (Fig. 6). Low-volatility oils help reduce the need to “top up” as a result of lower evaporative losses and reduced oil consumption.
 |
|
Fig. 6. Volatility (%) with SAE 5W-20 motor oils blended |
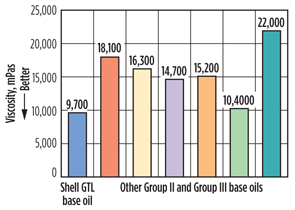
Low-temperature performance. To test low-temperature performance, viscosities of the different Group SAE 5W-20 motor oils were measured using the ASTM D4684 MRV TP-1 test method at −31°F. The oils that were blended with Shell GTL base oils demonstrated the lowest viscosity measured (Fig. 7). This indicates that motor oils blended with Shell GTL base oils have good performance at ultra-low temperatures (−13°F to −40°F) and flow readily through the engine under cold start conditions. Additionally, Shell GTL base oils allow for motor oil formulations to reach ultra-low viscosity grades, such as SAE 0W-30, SAE 0W-20 and SAE 0W-16.
 |
|
Fig. 7. Viscosity at −31°F with SAE 5W-20 engine oils |
Oxidation stability. Oxidation is the main cause of lubricant degradation and results in the breakdown of the lubricant due to extreme engine conditions. Oxidation stability of SAE 5W-30 motor oils formulated with different group base oils was tested by measuring the viscosity at −22°F, using the ASTM D7528 “ROBO” oxidation test and the percentage increase in kinematic viscosity at 212°F after the ASTM D7320 Sequence IIIG Engine test (Fig. 8). Shell GTL base oils offer good viscosity retention under severe oxidation tests and help maintain performance, even at very high and very low temperatures.
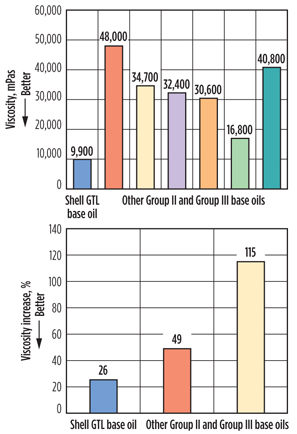 |
|
Fig. 8. Viscosity at –22°F after oxidation with SAE 5W-30 |
Future applications for base oils
Shell GTL base oils are now widely used in passenger car motor oils and have potential for use in other lubricants, such as heavy-duty diesel engine oils, transmission fluids, hydraulic oils and industrial oils, to name a few.
Heavy-duty diesel engine oils. The base oils have shown a significant crosshead wear benefit and a directionally improved injector-adjusting screw wear benefit over other Group III base oils in a low SAPS SAE 5W-30 formulation.4 Similar to motor oil formulations, significant improvement in piston cleanliness was observed.
Transmission fluids. The Brookfield viscosities measured at –40°C of Shell GTL base oils are better than those obtainable from other Group III base oils, delivering better low-temperature viscometrics.
Additionally, in transmission fluids, a number of frictional properties are normally optimized. Shell GTL base oils, being mainly isoparaffinic in nature, demonstrate good oxidative stability and high friction coefficients, providing benefits to transmission fluids.4
Hydraulic fluids. The lower density and high viscosity index of Shell GTL base oils lend themselves to potentially producing energy-efficient formulations by reducing inertial and bulk viscosity pumping energy losses and improving the thermal conductivity relative to other Group III base oils.4
Industrial gear oils. Gear oils are primarily base oils with very low additive treat rates. Improved base oil quality, such as that found in Shell GTL base oils, is highly desirable and manifests as an improved antioxidant response.4
Takeaway
Shell’s GTL process has enabled the production of high-quality base oils used in the manufacture of several passenger car motor oils ranging from products available to the general public to high-performance racing oils.
These base oils will also help in the creation of SAE 0W-16 motor oils for new US Corporate Average Fuel Economy regulations in the coming years, as Shell works to achieve 54.5 mpg with automobile original equipment manufacturers. GP
Literature cited
1Remans, T. J., G. Jenzer and A. Hoek, “Gas-to-liquids,” Handbook of Heterogeneous Catalysis, 2008.
2Fischer, F. and H. Tropsch, German patent 484 337, 1925.
3Geerlings, J. J. C., J. H. Wilson, G. J. Kramer, H. P. C. E. Kuipers, A. Hoek and H. M. Huisman, Applied Catalysis A: General, 1999.
4Wedlock, D. J., M. Lambert and T. T. Walker, “Gas-to-liquids base oils to assist in meeting OEM requirements 2010 and beyond,” 2nd Asia-Pacific Base Oil Conference, Beijing, China, October 2007.
 |
Sravani Gullapalli joined Shell in 2014 as an engineer in the lubricants discovery hub and is based out of Houston, Texas. Within the hub, Dr. Gullapalli works closely with the factory plant maintenance oil and alternative fuel compatibility teams as a project leader. Dr. Gullapalli graduated with a PhD in chemical engineering from Rice University in December 2013. In 2008, she earned her bachelor’s degree in chemical engineering from the National Institute of Technology in Karnataka, India.
 |
Allison Falender is the technology manager for Shell’s lubricants discovery hub. She has held multiple roles in biofuels and lubricants technology and has supported different product groups such as Pennzoil and Rotella. Dr. Falender’s team is a global multi-disciplinary group of scientists working on several projects that tie together innovation and product development. She earned her BA degree from Oberlin College in Oberlin, Ohio, with a double major in biology and sociology. She also holds a PhD in molecular and cellular biology from Baylor College of Medicine in Houston, Texas.




Comments