New inlet feed gas conditioning technology for contaminant removal in amine units
D. ENGEL, Nexo Solutions, The Woodlands, Texas and S. NORTHROP, ExxonMobil Upstream Research, Spring, Texas
Amine solvent foaming is a common challenge in gas treating plants, including those ahead of LNG manufacturing facilities. This can lead to reduced gas throughput, and in severe cases, solvent carryover and even plant shutdown. This foaming is ultimately caused by changes in the physical and chemical properties of the solvent. Chemical contaminants can lower amine solution surface tension and change surface rheology in such a way that foaming tendency is augmented, and foam is stabilized. Earlier work has shown that these contaminants are not restricted to liquid hydrocarbons but can be from a variety of other possible sources. While some of the contaminants may originate from within the amine unit itself, many are introduced with the gas, such as upstream production chemicals and produced water. While antifoam products can temporarily alleviate a foaming episode, they can also be a detriment and build up over time, fouling vessels and internals.
Some LNG plants employ inlet coalescers, filters or a water wash to remove contaminants in the feed gas. However, these systems may not be completely effective for the removal of certain contaminants. Furthermore, the vessels or water demands of these wash systems can be large and relatively inefficient. This article describes a new technology to prevent the introduction of contaminants into amine units. The novel system is installed in-line with the feed gas pipe and is comprised of a compact water-wash device capable of removing water soluble components such as surfactants and salts from incoming feed gas. The spent water can be treated and recycled to minimize water make-up and clean water consumption.
Gas treating for LNG manufacturing. Natural gas is treated to remove hydrogen sulfide (H2S) and carbon dioxide (CO2) to meet pipeline or other downstream process specifications. The removal of H2S and CO2—known as acid gases—to low specifications such as those for LNG production, demands much more from processing units. For LNG production, the H2S and CO2 specifications are usually 4 ppm and 50 ppm, respectively. Very few, if any, unprocessed natural gas streams contain such low levels of those acid gas contaminants. Consequently, most natural gas (including pipeline gas with 4 ppm H2S and 1%–2% CO2) used for LNG worldwide (approximately 244 MMtpy or ~22 Bft3d in 2015) must be treated, at least, for CO2 often using liquid reagents (also called solvents) such as alkanol amines. The chemistry of amine treating for CO2 removal is well known and documented elsewhere.
While many amine units function well over the life of the LNG facility, some are prone to periodic or even frequent foaming events. This article discusses likely causes and potential solutions to these foaming episodes in amine units, which also apply to gas treating in general.
Amine solvent foaming. When gas is routed through a liquid such as an amine solvent flowing across trays of an amine unit contactor (or regenerator), a short-lived, quick-breaking froth normally develops on top of the amine solvent liquid layer. If the gas bubbles or pockets cannot break the liquid-vapor interfacial structure quickly, they become encapsulated in the liquid phase and form what is commonly referred to as foam. Foam is essentially a collection of gas bubbles encapsulated inside a liquid film that will not easily coalesce or burst. Packed columns are less prone to foaming as upward flowing gas tends to flow across the surface of the downward flowing liquid instead of through the liquid layer on a tray. However, packed columns are not immune to liquid hold up and other symptoms of foaming, hence, the discussion below applies to them as well.
To better understand foam, one needs to consider two different aspects of it: foaming tendency and foam stability. Foaming tendency refers to the ease with which liquid film will encase gas bubbles. There is not a completely standardized measure for foaming tendency. However, a relative measure can be obtained by testing a fixed volume of amine solvent in a graduated cylinder and flowing air or nitrogen through a porous frit at set rate (100 ml/min), then measuring the level of foam generated.
Foam stability is related to the elasticity of the liquid layer around the gas bubble and the ability of the film to resist rupturing. Each gas bubble has an interfacial layer or “skin” that confers resistance and elasticity (hindering coalescence and structural collapse), enabling the liquid film around it to flex as the gas bubble deforms, expand, or contracts. A rough measure of foam stability can be obtained using the test just described by turning off the gas flow and determining the amount of time (in seconds) it takes for the foam to completely break. This is also expressed as foam break rate.
FIG. 1 shows an apparatus used to test foaming in amine solvents. The column has a bottom porous glass frit for gas dispersion. Interestingly, some amine solvents display high foam tendency, but the foam is short-lived once gas is shut off, and break rates are fast. In other cases, the foam tendency is not as high; however, the foam stability is strong with slow break rates. The worst scenario is an amine unit with a solvent that has both high foam tendency and strong stability.

FIG. 1. Example of a foam test column showing an amine solvent with significant foam tendency.
When an amine unit circulates a solvent that displays foaming tendency and foam stability, foam is initiated when process perturbations occur beyond what the unit can tolerate. A decrease in surface tension will sometimes correlate with an increased foaming tendency of the solvent, such as when liquid hydrocarbons are introduced into the system. However, this foam can be short-lived, and in many cases, goes unnoticed. Surfactants and other organic compounds can increase the foaming tendency as well as foam stability. When this type of foaming occurs, it does not go unnoticed and several process changes may be observed, such as:
- Differential pressure increases across the contactor and/or regenerator
- Decrease in contactor and/or regenerator bottoms liquid level
- Temperature bulge position changes inside the contactor tower
- Increasing liquid level in the amine contactor after-scrubber, as amine solvent is carried over with the treated gas (leading also to amine solvent losses)
- Increase in H2S in the treated gas (and CO2 for LNG cases)
- Amine contamination of the regenerator reflux water.
Note: The mechanical damage to tower internals or excessive gas velocity inside the amine unit contactor (or regenerator) can result in tower flooding and mechanical frothing/foaming which is distinct from pure chemical foaming. However, the symptoms are similar to those described above, so it can be difficult to distinguish foaming from flooding based on operational observations alone.
In a severe flooding-frothing-foaming event, the first action (after dosing antifoam into the system) is usually reducing the gas rate to lower the differential pressure across the contactor and allow any held-up liquid to fall into the bottom of the contactor tower. It can take a substantial reduction in the gas rate to recover control of the system. In addition to the reduction of feed gas to the amine unit, the upset must work itself through the rest of the unit and may require further operator intervention such as flash tank and regenerator adjustments. If frequent, these foaming events can measurably reduce the output of the LNG production facility.
Foaming of the amine solvent can often lead to carryover from the contactor or regenerator caused by entrained liquids with the treated gas or acid gas, respectively. Most amine units have separation vessels such as a knockout drum at the contactor outlet to recover most of the amine solvent carryover. If the amine unit is treating liquid hydrocarbon (e.g., condensate co-produced with gas), “foam” can be replaced by “emulsion” in the above discussion.
Any carryover from the amine contactor in the hydrocarbon liquid treating case is recovered by a water wash stage to remove any emulsified amine solvent present in the treated liquid hydrocarbon. In extreme cases, amine solvent carryover may reach downstream units such as dehydration plants, mercaptan removal plants or caustic treaters. Sometimes, the amine solvent carryover can infiltrate the main fuel gas system. Foaming in a regenerator is also detrimental as contaminated amine solvents often do not regenerate fully. Furthermore, carryover with the acid gas can reach the sulfur recovery unit, flare system or other downstream process.
Antifoam addition is a common method to temporarily control the deleterious effects of foaming. However, the effectiveness of a given antifoam may be limited as amine units sometimes use antifoam and experience little to no apparent foam reduction. Some plants use antifoams in the amine unit on a regular basis for short-term relief, but this can harm the solvent in the long-term. In fact, antifoams should not be used on a constant or daily basis. Root-cause analysis of foaming and the elimination of its source is the best way to deal with a foaming amine solvent. Nevertheless, antifoams may need to be used when sporadic foaming incidents occur and the source of foaming agent has not yet been identified.
Antifoams in amine units can accumulate on top of the solvent as a separate phase in the amine contactor flash tank or the regenerator sump, for example. Antifoams also can render activated carbon beds useless as they saturate the carbon and hinder entry of contaminants into the pore structure. Silicone antifoams are small droplets that are very effective for reducing foam (10x more effective than normal polyglycol antifoams). However, silicones should not be used in amine units because of their inherent physical incompatibility with aqueous solvents. Most silicone-based antifoams are removed using filters 10 microns or smaller.
Amine solvent foaming can usually be limited to a single root-cause: chemical contamination. However, the chemical contamination can originate from several sources. Clean amine solutions will not foam and should not froth. Hence, standard design and operation advice for amine units typically includes: 1) specifying efficient inlet solids and liquids separation systems to prevent ingress of most feed contaminants, 2) setting the lean amine temperature at least 10°F above the inlet gas temperature to avoid liquid hydrocarbon condensation in the contactor, and 3) including 15%–25% slipstream of carbon adsorption to adsorb soluble contaminants in the solvent. Additional measures include the sparing use of effective antifoams (applied efficiently), and lean and rich filtration to remove suspended solids.
Despite appearing to follow these empirical rules, some amine units still find themselves prone to foaming events. Therefore, it becomes a question of determining the source of solvent contamination and eliminating it. The following is a list of the most common sources of amine solvent chemical contamination:
- Ineffective inlet separation leading to contaminant ingress as solids or liquids.
- Sub-micron aerosols:
- Lubrication oils
- Produced water
- Surfactant-based chemicals from upstream treating such as corrosion inhibitors.
- Triethylene glycol (TEG) from upstream dehydration units is frequently detected in amine units, though TEG is not generally regarded as a foam promoter. However, TEG may react with amine at regenerator temperatures and thus has the potential of generating foam promoters.
- Gas-phase contaminants carried with the feed gas such as benzene, toluene, ethylbenzene and xylenes (BTEX), methanol, organic acids and especially pipeline chemicals such as corrosion inhibitors. Organic acids in a non-ionic form may have sufficient vapor pressure to be carried from upstream with the feed gas.
- Hydrocarbon condensation inside the amine unit contactor by not maintaining appropriated temperature differential between lean amine and inlet gas or by processing rich feed gas.
- Incorrect activated carbon (activated carbon exposed to phosphorous-based activation).
- Problems with activated carbon changeout (insufficient backwash to remove fines; post-filter passing fines into system).
- Activated carbon bed spent or carbon bed being bypassed.
- High concentration of suspended solids in the amine solvent (some solids can stabilize foam).
- High soluble iron in the lean amine (rapid solids formation in the contactor).
- Excess antifoam injection (excess antifoam use can induce foam).
- Incorrect antifoam (some antifoams will cause foam formation, especially if overdosed).
- Contaminants present in the fresh amine solvent and/or make-up water.
- In-situ generation of contaminants through oxidation or high regeneration temperatures.
It is important to emphasize that amine solvent foaming can be eliminated or greatly reduced in severity and/or frequency if efficient inlet separation and filter coalescence is in place upstream of the amine contactor. In addition, it is necessary to have sufficient amine solution filtration, effective activated carbon adsorption beds, and to correctly operate and maintain these units.
The physical chemistry of surfactant contaminants. Surfactants are interface-active molecules. They can have a wide range of molecular motifs. However, molecular surfactants usually consist of a polar section or head, and a non-polar group, often a hydrocarbon chain or tail (FIG. 2). The polar part of the molecule can interact with polar solvents like water, and it is therefore also called the hydrophilic portion. Conversely, the non-polar part can interact with non-polar materials such as hydrocarbons, and is therefore called the lipophilic or hydrophobic portion.
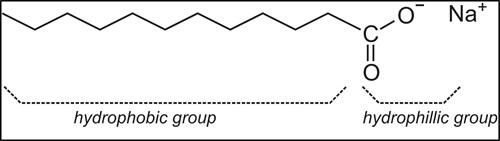
FIG. 2. An example of a molecular surfactant.
Molecular surfactants can be classified according to the charge of their polar head group:
- Anionic surfactants have a negatively charged head.
- Cationic surfactants have a positively charged head.
- Zwitterionic surfactants have a zwitterionic head group (both positive and negative charge).
- Non-ionic surfactants have an uncharged polar head group.
Surfactants adsorb preferably at interfaces where they find the most energetically favorable conditions due to their two-part structure. At a water surface, for example, the surfactants orient themselves in such a way that the head group resides in the water and the hydrocarbon chain points to the gaseous phase (FIG. 3). Thus, surfactants can "mediate" between two phases as they can form strong interactions with both of them. The interfacial tension consequently decreases since it is easier to generate more interfacial area when those molecules are present. The addition of surfactants facilitates the mixing of non-polar (in this case, gas) and polar phases. This phenomenon is used in the detergent industry, for example, to bring oil into the water phase.
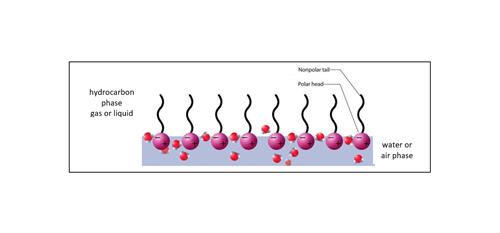
FIG. 3. Example of surfactants populating a hydrocarbon/water interface.
The decrease of the interfacial tension caused by surfactants accelerates as more surfactant molecules move to the gas/water interface. Once the interface is saturated, the addition of more surfactant will not decrease the interfacial tension any further. At this point, organized systems such as micelles may appear in the aqueous phase (FIG. 4).
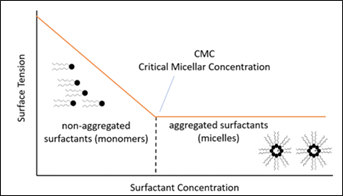
FIG. 4. Example of surfactants and its critical micelle concentration.
It is important to mention that solids such as iron sulfide (FeS) can, under certain conditions, also act as foam stabilizers because the solids’ surfaces can interact with both water and hydrocarbon (gas and liquid) simultaneously. FeS is particularly notorious for this because it tends to be in platelet form. For the purposes of this article, solid-based foam stabilizers (i.e., particles) will not be covered; only molecular surfactants as described previously will be considered.
Examples of surfactants commonly found in gas processing feeds, such as amine unit and dehydration unit feed gas, include lubrication oils and upstream process additives.2 Lubrication oils from gas compressors typically contain 90% base oil (most often petroleum fractions, called mineral oils) and about 10% additives for various functions, which often have surface-active properties. Additives that reduce friction and wear, increase viscosity, improve viscosity index, as well as improve resistance to corrosion, oxidation, aging and contamination in upstream processes also often have surface-active properties. Conversely, upstream process additives can be biocides, corrosion inhibitors, H2S scavengers, hydrate inhibitors or paraffin inhibitors, among others. Corrosion inhibitors (filming amines or quaternary ammonium salts with alkanol segments) are examples of process additives with surface-active properties. FIG. 5 shows how a filming amine performs, and a possible general molecular structure. As with surfactants in general, a filming amine has a hydrophobic section (long alkyl chain), and a hydrophilic section (polar ionic center).
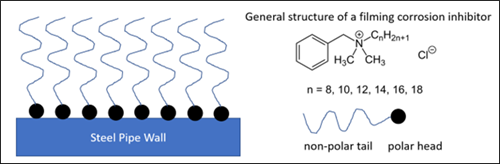
FIG. 5. An example of the mechanism of a filming surfactant such as a quaternary ammonium salt, cationic corrosion inhibitor (left). A general structure of a simple filming corrosion inhibitor (right).
FIG. 6 shows the change in surface tension of distilled water compared to distilled water when used as a scrubbing agent for surfactants. The decrease in surface tension from 72 mN/m (millinewtons/meter) to 46 mN/m is a clear indication of the surfactant’s presence. Similar effects are observed with some upstream process additives such as corrosion inhibitors. The decrease in surface tension is usually a good indicator of higher foaming tendency, but low surface tension alone does not mean that a liquid will foam. A pure liquid may have a low surface tension (e.g., hexane at 19 mN/m), but without a surface-active contaminant in the liquid, there is no mechanism for foam stabilization.
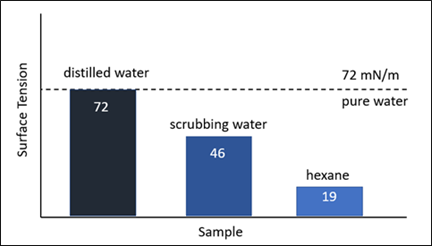
FIG. 6. Surface tension of distilled water and hexanes in comparison with water contacted with surfactant.
Note: heat stable salts can increase the surface tension of an amine solvent, and thus may cancel out the lowering of surface tension due to surfactant. Therefore, a working amine solvent may appear to have a normal surface tension value; however, it could have a substantial surfactant concentration, and hence high foaming tendency (FIG. 7).
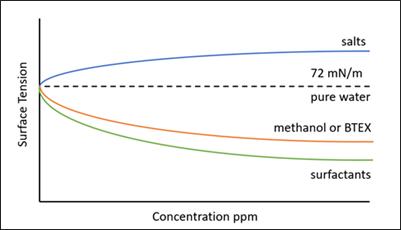
FIG. 7. Effect of contaminants on the surface tension of pure water.
A better, but not absolute correlative parameter to foam stability, is liquid surface dilatational viscosity. This has been measured using an oscillating bubble method. Despite several detailed studies, foam stability is not completely understood. However, part of what stabilizes foam is the ability of the surfactant to dampen surface tension perturbations when the liquid surface area changes. A cursory look at literature did not reveal any references that specifically examined the viscoelastic properties of amine solvent systems with or without contaminants. However, a project along that line has been suggested.
Minimizing surfactants in amine solvents. Excessive foaming can cause solvent carryover out of the contactor or regenerator. This can ultimately result in liquid contaminants appearing in downstream processes since separation devices (like demister pads) lose their separation efficiency as the liquid content of the gas increases. Poor phase separation can lead to multiple downstream impacts in addition to solvent loss. More importantly, foaming events can reduce treating capacity and consequently lower throughput of the LNG plant.
Inlet filter and coalescers. The first line of defense against surfactants is good inlet filter separation and coalescence to minimize aerosol carry-in to the amine. There are many upstream chemicals such as those in slickwater fracking fluid, compressor oils, amine corrosion inhibitors and others, that can contaminate amine and increase its foaming tendency and stability.
Lean amine temperature. Though heavy hydrocarbons do not have polar heads as described above, they do seem to act as surfactants in amine solutions. To ensure that they do not condense in the amine absorber, standard practice is to run the lean amine at least 10°F above the temperature of the inlet gas.
Amine carbon adsorption. If surfactant contaminants do make it into the amine system, a carbon bed may be used to remove them from the solution. The usual arrangement is to take a slip stream of lean amine and run it through a mechanical filter, followed by a carbon filter, and then another mechanical filter. The slip stream is often 10%–20% of full flow; however, a minimum of 25% is recommended with 15 min contact time. In many cases, activated carbon can remove surfactants from the amine solvent; however, it can take time to effectively remove the contaminant.
In some cases, activated carbon can only partially reduce foaming. In these cases, other materials can be used for foam contaminant removal and amine purification. Some novel adsorbents—such as the dual adsorbent NF-75—can remove foam when all other materials fail. FIG. 8 shows the dual mixed-bed material used (left) for the process and the resulting comparison (right). It can be clearly observed that foam was reduced, and the amine solvent was also clarified. This material can be used in all existing activated carbon bed vessels.

FIG. 8. Dual adsorbent NF-75 material used for foam reduction and amine clarification (left). Amine solvent presenting foam (right) before and after treating with the proprietary adsorbent NF-75.
Despite having these three standard protocols for reducing amine contaminants and their effects, some amine systems still exhibit substantial foaming tendency. Does this mean that some amines have inherent foaming tendency? Or can there be another mechanism for surfactant transport to the amine?
Organic acids: The Trojan horse? For the ionic surfactants described above, the only way for them to be transported to amine is via aerosol. Charged molecules cannot travel in the gas phase. However, neutral molecules can. An example is acetic acid, the main component in vinegar. The reason one can detect its odor is because it is transported in the gas phase into one’s nose. The protonated form of the acid can dissolve in aqueous solutions where it can dissociate into ions. This is particularly true if the aqueous phase has elevated pH, as does amine.
The foregoing situation would apply to most organic acids present in the hydrocarbon reservoir. For sour gas reservoirs (with low pH water), protonated organic acids can vaporize and be transported in the gas phase through the filter coalescer. They could then be captured in the amine contactor and become anionic surfactants.
Water wash. If conventional separation and filter coalescence are not completely effective at removing incoming aerosols and surfactants, water washing likely will be. A typical water wash tower consists of 3 or 4 trays (or decks) and employs countercurrent flow of water (downward) relative to gas (upward). If there is appreciable acid gas (H2S and/or CO2) in the feed gas, the wash tower shell will likely be fabricated from stainless steel to avoid corrosion. The possibility of carryover of wash water through the downstream filter coalescer to the amine unit discourages the use of corrosion inhibitor in the wash system.
The vessel and associated water demand of these wash systems can be relatively large, and thus difficult to justify in the plant design if vapor-phase surfactant intrusion is not a known problem in that region. What is needed is a compact system that performs an effective water wash, but consumes little excess water, and can be easily retrofitted into an existing amine facility. The following are two novel water wash systems that can be installed in-line with the feed gas pipe and are comprised of compact water-wash devices capable of removing water soluble components such as surfactants and salts from incoming feed gas. The spent water can be treated and recycled to minimize water make-up and clean water consumption.
The novel water wash systems can be utilized separately or in combination. Both proprietary contactors are depicted in FIG. 9. One of the proprietary contactors (cMIST™) is a co-current gas-liquid contactor that has been successfully applied to gas dehydration with TEG and is being tested for selective H2S removal. The other proprietary contactor (Exion®) has been used for removing H2S and mercaptans from liquid streams; however, it can also be used for surfactant removal from feed gas. These systems can also be used in combination to effectively remove virtually all soluble gas phase contaminants from the feed gas stream. The second contactor (Exion®) can also have a water reuse stage that allows the water to be reused by removing any surfactants from the spent water.
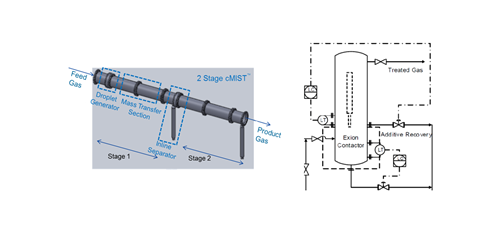
FIG. 9. Two different proprietary contactors: cMIST™ contacting device (left), and an Exion® device (right).
For the combination case, water would be injected upstream of the Exion device, where it would be coalesced and collected. That “polishing” water would pump upstream to the cMIST™ unit, where it would be sprayed into the gas steam and a short mass transfer zone, with the liquid being recovered by an inline separator. A small amount of the recovered water would be bled off, while the remainder (with a little clean makeup water) flows to a small mechanical and activated carbon filter.
In addition to absorbing water-soluble components, the water droplets may coalesce with other aerosols to make them easy to capture and recover from the gas stream. The cleaned gas then flows to the amine unit.
Production and pipeline chemicals: The hidden foaming agent? Recent field tests using a gas slip stream and a test unit equipped with a water wash to scrub the contaminants from the gas revealed that often production chemicals are vapor-borne and carried with the feed gas into the amine unit. The gas phase contaminants caused the wash water to have a significant foaming tendency and considerable stability. Laboratory testing of common corrosion inhibitors added to 50% methyl diethanolamine (MDEA) caused significant foaming. FIG. 10 summarizes the laboratory test performed to screen various corrosion inhibitors promoting amine foam. All tested chemicals produced foam given the foam column height. The foam stability was also significant with some chemicals stabilizing foam for several hours.
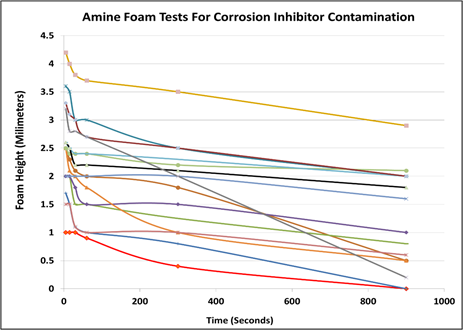
FIG. 10. Foaming tests of laboratory grade 50% MDEA in distilled water with added corrosion inhibitors. Each line represents a different corrosion inhibitor used in pipelines. The red line represents the control sample containing no corrosion inhibitor.
To support the hypothesis that surfactants such as corrosion inhibitors can be carried in the vapor phase with the gas stream, a simple but important experiment was conducted. The experiment consisted of passing air over the surface of water containing a known surfactant-based corrosion inhibitor in a glass bottle (FIG. 11). The air flow was passed over the surface of the water phase with no possibility of aerosol formation. The air was then routed into another bottle containing distilled water only. The air stream was bubbled into the distilled water to scrub any gas-phase components.
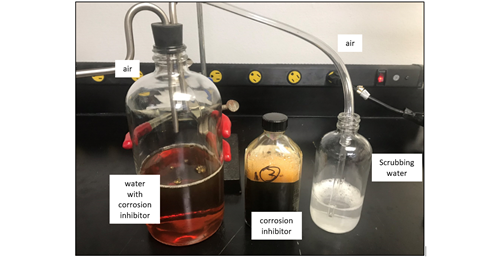
FIG. 11. Set-up to test carryover of gas phase corrosion inhibitors and scrubbed with distilled water. The corrosion inhibition pipeline chemical is shown in the center and is a significant foam promoter.
Initially, the experiment did not reveal any appreciable foam formation. However, after ~6 hours, a small stable froth head was observed at the surface of the scrubbing water reservoir. This froth was short lived but indicated that the water was starting to be contaminated with components that can stabilize foam. After 12 hr, the froth became stronger and more stable leading to the initial stages of foam. The surface tension of the water decreased about 9% compared to distilled water. This was clear evidence that gas phase surfactants can travel in the gas stream and likely penetrate any filter and coalescer before ingression into an amine unit or glycol unit. Filters can remove solid particles, while coalescers can remove liquid droplets or aerosols. However, gas phase components will not be removed by either method. Hence, the need for a water wash system to greatly reduce the chance of surfactant carry-in to the amine system.
Takeaways. The following are several takeaways from this article:
- Virtually every natural gas stream with H2S or CO2 should be treated by amine or other solvents to meet gas specifications for pipelines, LNG or other cryogenic processes.
- Solvent foaming tendency and foam stability are complex phenomena in both amine and glycol units, and generally originate from multiple sources. The foaming of amine and glycol solvents has not been thoroughly investigated, largely because such a wide range of factors and variables present in actual processing units making its understanding difficult.
- Increased foaming tendency is usually the result of lowering surface tension by organic components such as certain solvents, hydrocarbons and specific surfactants that enter the unit. However, surface tension alone cannot always explain foam tendency or foam stability because of the multiple contaminants present in amine or glycol solvents that can increase surface tension. More complete surface rheology tests must be conducted to determine foam film elasticity and better correlate to foam formation.
- Foam stability is attributed to contaminants that can stabilize foam, primarily surfactants. These form elastic films capable of self-assembling at critical interfaces, leading to foam stabilization.
- Compact water wash upstream of processing units may be an effective means of absorbing gas phase contaminants and, by implication, reduce foaming tendency and stability in the downstream amine unit.




Comments