The race for clean energy: LNG and LH2 as the next frontier in fuel technology
A. LODHI, Wood Plc., Dubai, UAE; M. M. AMMAD, AIN Engineering Services, Karachi, Pakistan; and M. SHAHZAIB, Royal Vopak Netherlands, Rotterdam, Netherlands
Liquefied natural gas (LNG) and liquid hydrogen (LH2) are considered as promising potential fuel sources for transportation, industry and power generation. While both fuels have attained significant attention in these areas, both have properties that requires specialized storage and transportation infrastructure. LNG is primarily composed of methane (CH4) and can be utilized as an alternative for diesel and gasoline in transportation and power generation. It is presently a widely used fuel in various industries and transportation sectors due to its high energy density, established infrastructure and lower cost compared to LH2. Conversely, LH2 is gaining attention as an impending fuel for the future due to its renewable source as green H2, high energy efficiency and zero-emissions properties.
This article presents a comparison of LNG and LH2 as fuels, and discusses the properties and characteristics of both, including energy density, transportation, storage, safety, environmental impact, availability and cost. A radiation analysis was also performed and compared for both fuels to compare the safety requirements associated with their storage and layout. While both fuels show promise in reducing emissions, the challenges and choice of fuel will depend on the specific application, economics and the availability of the fuel. LNG is presently more widely available and has a more established infrastructure, making it a more practical choice for many energy applications. However, as the demand for low-emissions fuels increases, LH2 may become a more viable option in the future.
LNG can be considered a transitional fuel, providing a robust pathway towards a sustainable energy future. LNG projects can be quite complex and require significant capital expenditure, with numerous regulatory requirements that must be met. However, by following a rigorous project development process, conducting thorough environmental and social impact assessments, engaging with stakeholders and collaborating closely with regulators, LNG projects are built with minimal impact on the environment and surrounding communities.
Similarly, H2 is seen as a potential alternative to fossil fuels, offering a clean and sustainable solution that reduces carbon footprint. The use of H2 as fuel in the industrial and transportation sectors will reduce the dependence on depleting oil and gas reserves globally and will allow the production, storage and utilization of energy in abundance from locally available resources, considerably reducing emissions.
The combustion of H2 generates energy and water without producing carbon emissions, providing an effective option for the energy transition. However, energy is needed for H2 production, as H2 does not exist in free form. In this regard, green H2 is the cleanest option as it is produced from renewable energy sources.
To liquify H2, its operating temperature must be reduced to –253°C, which requires extensive energy. In comparison, natural gas is liquified at –163°C, so the liquefaction process for LNG is more economical with respect to energy consumption. Because both are liquefied and, therefore, reduced in volume from their original state (TABLE 1), storage and transportation are more economical.
| LH2 | LNG | |
| Density, kg/m3 | 71 | 424 |
| Lower heating value, MJ/kg | 120 | 47 |
| Energy density*, MJ/l | 8.52 | 22.2 |
| Storage temperature, °C | –253 | –160 |
*These values vary depending on the pressure, temperature and composition of LNG and the purity of H2.
TABLE 1. The respective storage temperatures and atmospheric pressures of LNG and LH2
To further compare LNG and LH2 as fuel, the following aspects are discussed in detail.
Availability. The global availability of natural gas is significant—natural gas has been a key energy source for many countries. However, its availability is subject to geographical locations. Conversely, H2 is an elemental gas that does not occur naturally in large quantities. It is often produced through various methods, such as the steam methane reforming (SMR) of natural gas, the electrolysis of water using renewable electricity and other processes. H2 availability is presently limited compared to natural gas due to the challenges of large-scale production and a lack of distribution infrastructure. However, there is growing interest in H2 as a clean energy carrier and a potential solution for decarbonization efforts. Research and investment in H2 technologies are ongoing to enhance its availability and accessibility.
Storage. LNG is stored in large, insulated tanks at atmospheric pressure, where it is kept at a temperature of around –160°C. In contrast, LH2 must be stored at an extremely low temperature of –253°C to remain in liquid form. The storage of LNG and LStorage and transfer pipelines are designed with insulation, but this does not perfectly contain heat and prevent leakage, generating boil-off gas (BOG) that must be handled—a typical method of managing BOG is reliquification units. The BOG rate for liquid H2 is higher than LNG, depending on the type, size, shape and design of the storage tank, insulation and ambient conditions.
The type of storage tanks used for LNG and liquid H2 are similar in that they are both typically double-walled, vacuum-insulated tanks designed to maintain the low operating temperatures required for these fuels to remain in liquid state. Additional high-grade insulations are required. The inner wall of LNG double-walled tanks is typically made of 9% nickel steel, whereas for LH2 it is made of aluminum alloy, austenitic stainless steel or 36% nickel steel to withstand the low operating temperature.
Safety. LNG is a flammable gas that can ignite if it comes into contact with an ignition source like a spark or flame. LNG has a flammability range of approximately 5%–15% by volume of air. In contrast, LH2 can ignite at low concentrations of air and burn with an invisible flame, as it has a wide flammability range from 4%–74%. Furthermore, LH2 has a very low ignition energy (0.017 MJ)—this implies that even a small spark or heat source can ignite LH2, making it potentially more hazardous than LNG.
A primary safety hazard linked with LNG and LH2 is the risk of spillage during transportation or storage. A leak in the storage or transportation system can vaporize and create a flammable or explosive atmosphere. Also, both are stored at exceedingly low temperatures, which can cause frostbite or cryogenic burns if they come into contact with skin or other materials.
A radiation analysis was also performed for both fuels (FIGS. 1 and 2), maintaining a similar leakage scenario at their operating storage conditions: it was determined that radiation contours for LNG as a result of fire are more severe than LH2. The following factors were considered when performing radiation modeling:
- Stability class: D
- Hole size: 2 in.
- Relative humidity: 70%
- Wind speed: 5 m/sec
- Ambient temperature: 30°C.
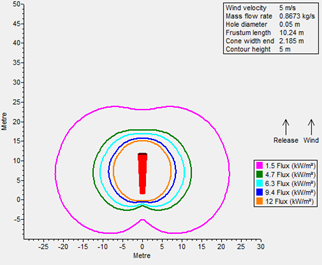
FIG. 1. Thermal radiation contours for LNG.
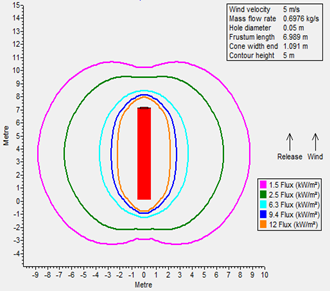
FIG. 2. Thermal radiation contours for LH2.
Energy density. As shown in TABLE 1, at their respective storage temperatures and atmospheric pressures, the energy density per unit volume of LNG is higher than that of LH2, which implies that LNG can store more energy than LH2 for the same volume or space, making it a better option where storage space is compact. Additionally, in fuel carriers and ships, more fuel in terms of energy volume per unit can be transported via LNG compared to LH2. In terms of mass, the energy density of LH2 is greater than LNG; however, a greater volume of LH2 is required to attain the same amount of energy as LNG. H2 may be transported in the form of ammonia and methanol, as they have better energy density.
Transportation. LNG and LH2 can be transported via large tanker ships, tankers, pipelines or cylinders—the most common methods adopted worldwide for bulk transportation are pipeline and tanker ships. Transportation of LH2 through pipelines is more problematic compared to LNG due to the exceedingly low temperature and high rate of heat transfer of the liquid. LNG is also transported in the form of gas at ambient temperature through pipelines, converted at terminals and jetties to gas through vaporizers or water bath heaters, connected to the gas network and then utilized as natural gas at its end use.
Due to the challenges involved in transporting LH2 through pipelines, it is usually only done over short distances, such as from a production site to a nearby storage facility or end user. In contrast, LNG can be transported through pipelines over longer distances, making it a more practical choice for transportation between different regions or countries via pipeline. The pipelines are heavily insulated to inhibit heat transfer and reduce the rate of evaporation.
Pipeline insulation. While transferring LNG and LH2 from ship to shore and vice versa to terminal, one challenge is to sustain the cryogenic temperature of the liquid inside the pipeline and minimize heat losses by utilizing efficient and safe thermal insulation. The pipelines must be insulated to prevent heat transfer and minimize the rate of evaporation of the liquid. The materials and insulation used for LNG and LH2 pipelines are selected based on their ability to withstand the specific properties of these fuels, as well as other factors that include cost, availability and ease of manufacturing. LNG pipelines are typically coated with closed-cell polyurethane foam or cellular glass insulation to help retain the low temperature of the fuel during transportation, while LH2 pipelines are often coated with high-density multi-layer insulation (MLI) to maintain the cryogenic temperature of less than –253°C inside the pipeline.
Environmental impact. As a fossil fuel, LNG releases greenhouse gases (GHG) when burned, such as carbon dioxide (CO2), CH4 and nitrous oxide (N2O). In contrast, LH2 is a cleaner fuel that only emits water vapor when burned.
The production of LNG entails energy and releases GHG. LH2 also requires energy for production, and the emissions depend upon the source of that energy. If the energy originates from renewable sources like solar or wind, the emissions will be minimal.
Both fuels produce fewer emissions than traditional fossil fuels, such as gasoline or diesel. However, LH2 produces no emissions when burned, making it a cleaner option for transportation and energy generation.
LNG is a mature technology, with a well-established supply chain and infrastructure. Natural gas prices, which change based on supply and demand, are a major factor in determining the cost of manufacturing LNG. Comparatively, the cost of producing and storing LH2 is still somewhat high because it is a relatively new technology. The production of LH2 requires a substantial quantity of energy that contributes to its high cost. Moreover, specialized infrastructure and equipment are needed for the transportation and storage of LH2, again adding to the overall cost.
Nevertheless, the cost of producing LH2 is anticipated to decrease as more advanced technologies are developed and economies of scale are achieved. LH2 has the potential to be produced using renewable energy sources, such as wind or solar power, which could reduce its cost over time.
Takeaway. Overall, LH2 has the potential to be a cleaner and more environmentally friendly fuel than LNG, but it also requires a significant amount of energy for production and infrastructure development. The cost of H2 production is expected to decrease in the future as more advanced technologies are developed, making it a more viable choice for transportation and energy generation.
ABOUT THE AUTHORS
ASAD ASHFAQ LODHI is a Senior Process Engineer with Wood Plc. in Dubai, UAE. With 12 yr of professional experience in process engineering, Lodhi has several articles published in the field of oil and gas. He holds a B.E degree in chemical engineering and an MS degree in project management.
MUHAMMAD MUSAB AMMAD is a Principal Process Engineer with AIN Engineering Services. He has worked mainly in the oil and gas processing segment with hands-on experience in plant design and optimization. Ammad earned a B.E degree in chemical engineering.
MUHAMMAD SHAHZAIB is the Global Safety, Health and Environment (HSE) Manager for Royal Vopak Netherlands and has extensive experience in operations and SHE management in the chemical storage and handling industry. He specializes in operations and the safe handling of cryogenic chemicals. Shahzaib holds an M.E. degree in chemical engineering.




Comments