The heat is on: Improving vaporization and corrosion control for natural gas chromatography
D. Dickerson, AURA Gas Controls, Virginia Beach, Virginia
With the rise of global demand for natural gas in tandem with the increase in value of NGL, producers are extracting, processing and transporting larger varieties of hydrocarbons. Global NGL production has expanded by 4% over the last 7 yr, bringing production to more 13 MMbpd,1 with the US, Saudi Arabia, Russia, China and Canada being the largest producers.2
Once extracted, the raw natural gas must undergo a multistage refining process to remove contaminants, such as sand, water, hydrogen sulfide (H2S) and any other unwanted compounds. To produce commercial-grade natural gas (a C1 hydrocarbon), the remaining hydrocarbons must be separated, and are then sold separately as feedstocks to the petrochemical industry.
This process involves several points of custody transfer, demanding accurate gas measurement to determine the quantity and quality of product being transferred, as well as the composition of the gas stream during processing and separation.3 In meeting natural gas measurement challenges with existing technology, natural gas chromatographs (GC) using thermal conductivity detectors (TCD) have emerged as a primary means of measuring energy content, product quality and numerous other parameters during custody transfer and processing, due to their accuracy and reliability.
TCD gas chromatographs separate analytes, based on the identifiable characteristic of thermal conductivity, as a heated, conditioned sample passes through a series of columns using a non-targeted carrier gas, such as helium, hydrogen or nitrogen. The GC identifies the analytes using an electronically heated filament mounted in a temperature-controlled cell. It quantifies the change ratio of thermal conductivity in the presence and absence of the sample as compounds separate at different rates through the equipment’s columns.4
However, measuring samples containing heavier hydrocarbons up to C5 (such as propane, butane and pentane), along with sour gas samples containing H2S and mercaptans, has created a challenge for those utilizing natural gas GCs. Accurate analysis demands that the sample be in the gaseous phase and within a precise pressure range, requiring an effective method of sample vaporization and pressure control while simultaneously providing corrosion resistance from sulfides and mercaptans.
The crux of effective natural gas sampling centers on the system’s ability to rapidly and wholly vaporize large volumes of hydrocarbons, placing primary emphasis on the ability to produce high temperatures in a short amount of time. The traditional method of sample vaporization for analysis requires the use of an electronically heated vaporizing regulator to maintain the gas phase of the sample and flash any remaining liquids into the gaseous phase at the sample tap. This method ensures that a single phase of matter reaches the analyzer.
However, pressure increases as gases are vaporized, making it vital that the equipment incorporate a stage of pressure reduction to provide the final line pressure to the analyzer. The standard vaporizing regulator design uses a heated, straight flow path from the bottom inlet port to the seat of the regulator. When working with heavier hydrocarbons (C3 and higher), this method becomes problematic. The sample is minimally in contact with the heated surface, requiring the user to oversize the heater and drastically increase the temperature setting of the equipment (up to 200 W and 380°F) to provide the heat required to vaporize a sample of C1–C2 hydrocarbons.
Recent technological advances have been developed for regulator heating methods to mitigate the limiting heat effects exhibited by the traditional design. Fig. 1 shows the flow paths of the historical design using a straight flow path, and that of the modern method using a nonlinear flow path. The nonlinear method elongates the flow path and increases the duration for which the sample is in contact with the heated surface. This supports the optimal vaporization of hydrocarbons, and ensures that a representative sample is registered at the GC by eliminating the tendency of samples to separate into both gaseous and liquid phases in the presence of insufficient heat under the traditional method. Given that the sample is forced to receive longer heat duration, the regulator’s heater and temperature settings can be reduced to provide longer component lifecycle by limiting the rigors placed on the electronics.
|
Fig. 1. Technological advances in regulator design (left) utilize a nonlinear flow path to mitigate limiting heat effects, as illustrated in the traditional method (right). |
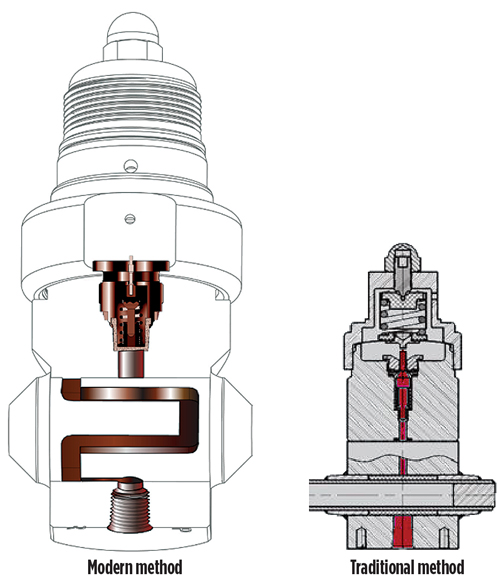 |
This same dynamic enables the vaporization of C3–C5 hydrocarbons in wellhead sampling applications and NGL processing. The modern method, which uses a nonlinear flow path, allows the equipment’s heater to fully traverse the heating surface. With a traditional design, the heater can be installed only partially into the stainless steel (SS) heating surface to avoid intersecting with the straight flow path. As a result, the regulator’s warmup time with the modern method is drastically reduced, allowing samples to be gathered faster than in the traditional method.
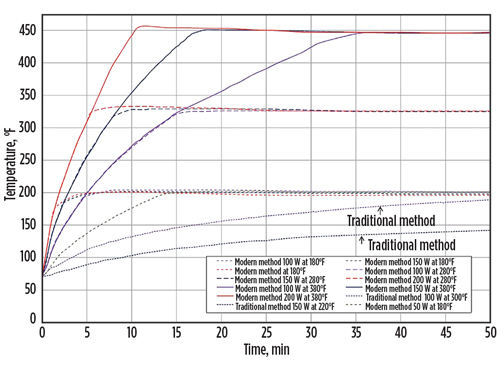
Fig. 2 shows a comparison of the heat produced over time between the modern and traditional methods of heat production. With a faster and more efficient heating method, the modern design produces more total heat and is capable of reaching temperatures up to 450°F. These parameters provide the necessary process conditions to vaporize heavier hydrocarbons that traditionally recondense into the liquid phase with insufficient heat, and across the pressure drop of a restricted orifice, like the seat of the regulator.
|
Fig. 2. A comparison of modern and traditional methods of heat production demonstrates that the modern method is more efficient in providing the necessary process conditions to vaporize heavier hydrocarbons. |
 |
To counter the potential for recondensation, the orifice size of the seat in the traditional method must be reduced to effectively limit the flowrate to the analyzer, so that the sample can maintain contact with the heated surface. Given that the modern method can produce higher temperatures in a shorter time span, the orifice size can be increased to produce a higher flowrate while still ensuring full sample vaporization. This increase becomes crucial in processing plants employing multiple GCs, as the facility can supply multiple analyzers from a single sample tap, rather than using a dedicated vaporizing regulator for each analyzer.
While the modern method of vaporization eliminates dual-phase sampling and supports applications using various types of hydrocarbons, it is also paramount that the equipment simultaneously provides the durability to mitigate corrosion from sour gas samples that are high in sulfurs and mercaptans.
The customary method of enabling accurate sampling of high-sulfur-content natural gas incorporates system components that are either constructed of exotic alloys high in nickel content to reduce corrosion, or that have amorphous, silicon-based coatings to render the surface of the equipment inert. However, recent technological advances in materials have emerged, providing superior replacements to the existing methods. Carboxysilane materials now simultaneously offer the chemical inertness and corrosive resistance required for low-ppm and percentage-level H2S measurements.
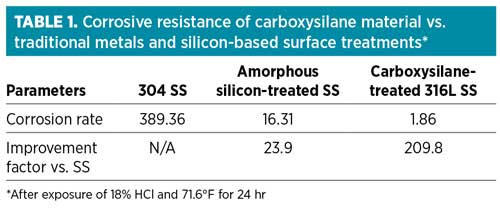
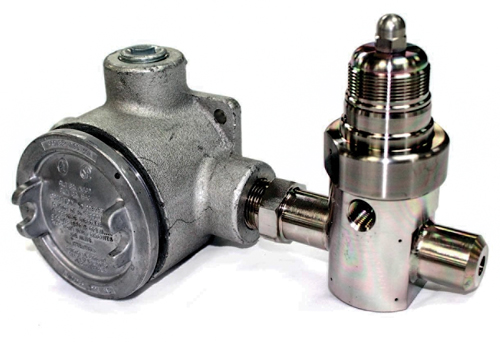
Table 1 illustrates the corrosive resistance of carboxysilane materials of construction vs. traditional metals and silicon-based surface treatments after 24 hr and exposure to 18% HCl. Fig. 3 shows a carboxysilane vaporizing regulator. Carboxysilane materials bond in depth with the crystal lattice of an SS substrate, as the deposition of carbon, oxygen and silicon achieve a flexible and wear-resistant, yet inert, surface that provides more than 200 times the corrosion resistance of untreated SS, and almost 10 times that of amorphous silicon coatings.5
 |
|
Fig. 3. A vaporizing regulator’s carboxysilane materials bond with the crystal lattice of an SS substrate to provide improved corrosion resistance. |
 |
The application of carbon and oxygen to the substrate increases the hardness of the material, mitigating corrosion from high-percentage concentrations of H2S and mercaptans as they react with the high moisture content in wet gas samples. Likewise, the carboxysilane construction material safeguards the regulator from component degradation. This type of degradation arises from the ambient environment; many natural gas sampling systems are located in difficult processing environments or in remote locations that are subject to the elements.
Similarly, the inherent moisture retention exhibited by SS proves problematic, as it is more difficult to dry the system. The carboxysilane’s deposition of silicon is vital to controlling moisture in the chromatography system and reducing cycle times, as the material will not absorb moisture or low-ppm mixtures. Moisture absorbed into the system often creates false analytical results, as compounds being monitored are either (1) absorbed into the moisture and not registered with the sample, or (2) new mixtures can be produced that create an unintended sample composition.
Relative samples can be registered significantly faster than systems using SS components through the reduction of dry downtimes and the corresponding increase in cycle times. Therefore, it is vital to sampling efficiency to use an effective method of vaporization in combination with materials that provide the accuracy and durability demanded in GC systems.
In addition to GC applications used to measure the properties of hydrocarbons, corrosion, time and temperature play similar roles in affecting the efficiency and accuracy of analytical instrumentation systems across multiple industries. Regardless of the hydrocarbon and analyzer technology used, the principles and techniques of vaporization discussed here can be applied in a general way to maintain the accuracy, reliability and durability of analytical instrumentation systems. GP
Literature cited
- International Energy Agency, online: http://www.iea.org
- U.S. Department of Energy, online: https://www.eia.gov
- Siemens Process Analytics, online: www.siemens.com
- Emerson Process Measurement, online: www.emersonprocess.com
- Silcotek, online: www.silcotek.com/whitepapers
 |
Dan Dickerson is the Channel Marketing Manager for AURA Gas Controls, with primary responsibility for the product development and marketing strategy of AURA’s global instrumentation solutions for the oil and gas, petrochemical, power generation, biotech, pharmaceutical and aerospace industries. Mr. Dickerson received a BS degree in innovation and technology management from Virginia Tech in Blacksburg, Virginia and an MBA degree from Saint Leo University in St. Leo, Florida.




Comments