DME technology advances provide clean alternative to diesel
R. G. Vedhara, Aum Energy Pte Ltd., Singapore
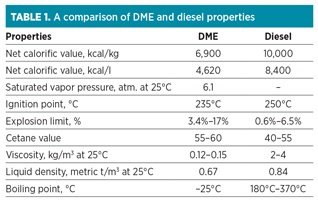
While dimethyl ether (DME) might sound like an exotic chemical, it is, in fact, the simplest ether compound, with a chemical formula of C2H6O. DME is a clean, colorless gas at ambient temperatures, but it can be liquefied under moderate pressure. This makes DME quite similar to propane and LPG for handling purposes (Table 1).
 |
DME has been used for decades in the personal care industry, mainly as an environmentally benign propellant in aerosols, as DME is nontoxic and is easily degraded in the troposphere.
The advantages of DME are many:
- Readily liquefies at 6 bar or –25°C
- Its transportability as a bulk commodity, which enhances trade
- Burns with a visible flame
- Degrades readily in the atmosphere
- Light odor; it smells faintly like ether
- Highly soluble in water and hydrocarbons
- Extremely low toxicity; zero environmental hazards
- Soot-free clean combustion/exhaust gas
- Renewable fuel; syngas can be made from biomass.
The potential of DME as an advanced clean fuel and chemical intermediate was recognized and developed by Amoco Corp. beginning in 1990. DME was seen as an attractive tool to monetize stranded and distressed gas resources with multiple promising markets, such as LPG blending, power generation, clean diesel, olefin and gasoline production, and more.
China was the first country to use DME as a fuel on a large scale. Its environmentally friendly characteristics helped accelerate usage at the turn of the century. China is now the largest producer of DME in the world at over 4 MMtpy. Approximately 50% of all methanol produced in China goes into DME, and around 90% of all DME produced goes into LPG blending, where DME is permitted as 20% of the blend on a weight basis. There are more than 60 DME manufacturing plants in operation across China that convert coal into syngas and then into methanol before dehydration into DME. Other plants have been built in the US, Sweden, South Korea and Japan, and a new plant is being built in the Caribbean, among other projects in the development pipeline.
While China’s focus on DME originated from its desire to create value from its vast coal resources, some companies believe that natural gas will become the predominant feedstock as DME technology is utilized in other markets. The abundance of natural gas around the world serves as both a clean fuel in its own right, and also as a competitive feedstock for conversion into other clean fuels, such as DME. Beyond LPG blending, DME can also be used as a clean diesel replacement, given its combustion properties and its lack of carbon-carbon bonds, which eliminates the production of particulate emissions.
A basic market offer is scaled at 11 MMscfd for the LPG blending market and 16.5 MMscfd for the diesel replacement market. These unit sizes produce 200 tpd and 300 tpd of DME, respectively, and also retain the option to produce methanol. Both these unit sizes are relocatable in the event of depletion in the underlying gas resource. As the market develops, projects closer to a world-scale 1 Mtpd are preferred, as the ability to generate a sustainable price advantage relative to diesel is significantly increased. Projects of this size will typically be located next to larger gas fields or gas pipelines to ensure feedstock security of supply. A gas-methanol-DME unit can be built in under three years, with all of the component parts already having been proven. With over 65 Mtpy of methanol being produced globally, the technology is well established and available off-the-shelf.
Underlying technology. The technical strategy is to build on the successful work done in the field in relation to the KOGAS 10-tpd demonstration facility in Incheon, South Korea (Fig. 1), and an enhanced reactive distillation unit for methanol-to-DME, which was successfully commissioned in the US in 2014.
 |
|
Fig. 1. A 10-tpd demonstration facility in Incheon, |
One of the unique features of the technical design is that only one product is being produced. All other products are recycled to extinction, thereby minimizing the environmental impact.
For many years, a significant quantity of process development work was conducted to commercialize a one-step approach. In this process, syngas is specifically produced to yield an H2/CO ratio of approximately 1. This syngas is then sent into a reactor where Fischer-Tropsch (FT) and dehydration reactions are conducted simultaneously, resulting in a reaction product that includes significant amount of DME. The DME is extracted out of the recycle loop along with CO2 and water.
In the two-step process, a syngas with an approximate H2/CO ratio of 2 is used to first make crude methanol. This is then catalytically dehydrated to yield DME. The benefit is that the technology involved in methanol production is well understood, and any process risks are related only to the small expenditure used for the dehydration reactor to make DME (Fig. 2).
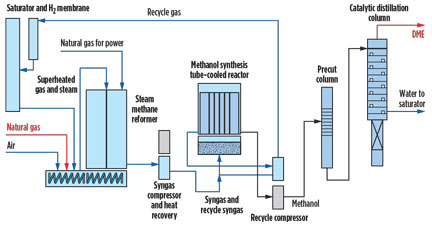 |
|
Fig. 2. A schematic of an efficient DME manufacturing plant. |
Modular technology and catalytic distillation. The largest portion of a modern methanol plant based on steam reforming to produce syngas is typically around 1,500 tpd. Above this capacity, autothermal reforming is the preferred methodology for making syngas. A 1,500-tpd methanol production facility is readily capable of making approximately 1,000 tpd of DME using dehydration technology, which, in turn, will typically comprise 3–4 trains of catalytic distillation columns.
It is recognized that plants capable of producing approximately 200 tpd–300 tpd must be designed and fabricated using modular technology. This ensures that significant cost savings can be generated in maintaining a tight supply chain and repeatable fabrication procedures. Smaller plants, by their very nature, will have slightly higher capital costs, but modular methodologies will mitigate this increase in capital, to some degree.
The specific advantage of catalytic distillation is that pure DME can be easily produced as a singular unit operation. The overhead reflux can be optimized to produce either propellant-grade or fuel-grade DME. An acid-based catalyst is used for dehydration of methanol to DME. This catalyst is loaded onto the distillation trays within the column, which is comprised of three sections: the lowest is the stripping section; the middle-section contains 15 to 20 trays loaded with catalyst; and the upper section is the rectification part.
DME can be readily made by the dehydration of methanol conducted over a heterogeneous catalyst. In the majority of cases, this catalyst is based upon an acidic formulation (Eq. 1).
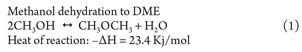
The catalytic distillation methanol dehydration process works under benign conditions. The operating pressure is typically in the range of 5 bar–10 bar, and the operating temperature is approximately 175°C at the bottom of the column and around 50°C at the top of the column.
The physical footprint required to produce DME from crude methanol is modest. A 200-tpd to 300-tpd DME production facility using crude methanol as the feed will require approximately 300 m2–400 m2, while a 1-Mtpd facility comprising 3–4 trains will require around 1,000 m2.
Concerns with the FT reaction. FT technologies are normally used to convert syngas into fuel-grade hydrocarbons. One of the problems with the FT reaction is what is commonly referred to as the Fleury distribution, which describes a phenomenon where this FT reaction produces a hydrocarbon product that ranges from C2 potentially up to C32. This wide range of hydrocarbon necessitates the use of several distillation columns to separate/fractionate the hydrocarbons to produce optimal products, such as gasoline and diesel. Much effort is directed toward stopping the Fleury distribution at a specific carbon number, such as C18. More diesel may be produced, but it will still require distillative separations, all of which significantly increase the costs associated with process units and offsites.
Processes to produce methanol result in the production of a singular product, which can then be the hub of other downstream derivatives. Methanol itself is a valuable and highly marketable commodity. In the process, a bulk of the waste heat is converted into medium-pressure steam that drives a steam turbine-based genset. The electrical power produced is used to drive the syngas compressors. This does result in a slightly higher capital cost, but also a significant drop in variable costs.
Vapor phase technology is the conventional method to conduct catalytic dehydration of methanol to DME. This low-pressure process operates at high temperatures in a vapor phase environment. One of the drawbacks of this approach is that multiple products ranging from light gases to unconverted methanol are produced. The DME must be separated from this product stream, requiring a number of operations and lending considerable complexity to the system. It is estimated that this approach would result in an approximate increase of 25% in capital costs. Published numbers indicate a significant increase in energy utilization, potentially as much as 15%–20%.
EPC and market considerations. From an engineering, procurement and construction (EPC) perspective, an increased competitive advantage in costs has been explored by working in conjunction with Indian companies. India’s cost base is substantially below that of Organization for Economic Cooperation and Development (OECD) markets. Concurrently, there is in-depth strength in the fields of bioprocessing and engineering, such as ethanol and brewing, providing options to secure professional services to lead the EPC effort for all 200-tpd to 300-tpd projects.
The central EPC role includes the coordination and management of the dozen or so package vendors involved in the unbundled procurement strategy. With an Indian cost structure underpinning the EPC size of the equation, DME is able to serve both OECD markets, as well as those of Africa and Southeast Asia, where an ability to mobilize teams to work under difficult and complicated conditions is paramount in project delivery, and where modularity and simplicity count.
This work has attracted interest from the upstream community, as well as from the World Bank’s Global Gas Flaring Reduction Partnership (GGFR), given the potential to optimize gas usage in areas where traditional routes to monetize gas are not attractive or where gas is flared. By providing a small-scale solution for the industry, flaring, which accounts for 2% of all greenhouse gas (GHG) emissions, can be reduced. In markets such as Indonesia and West Africa, where there is a shortage of LPG, DME can be readily absorbed into the local market with adherence to international guidelines on blending and usage, as established by the International Organization for Standardization (ISO) the American Society for Testing and Materials (ASTM).
Another advantage of using DME technology is that the process can readily absorb natural gas or biogas that is rich in CO2 as part of the conversion process, a key advantage for companies that struggle to place CO2 effectively. Ultimately, a simpler type of technology, such as DME, will lead to lower operational and maintenance costs, as well as more proficient operational performance.
A viable and clean diesel alternative. In Europe, North America and Asia, several companies have developed considerable interest in DME as a clean diesel alternative. Among these companies are automobile and engine manufacturers such as Volvo, Mack, Isuzu and Ford, which have announced a significant program to investigate the viability of DME in the passenger car segment.
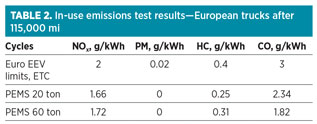
The emissions-related benefits of DME by Volvo’s European test results (Table 2) point to zero particulates and lower nitrogen oxides (NOx), zero sulfur oxides (SOx) and 10% lower carbon dioxide (CO2) emissions, but with equivalent fuel efficiency against a diesel engine.
 |
Isuzu has been developing DME since 2001, with a focus on city buses and short-haul trucks in tandem with a program to develop better diesel engines. The company states that DME for Euro 6 standards can be achieved with a simpler configuration than with a diesel engine:
- A simpler exhaust aftertreatment system (EAS) and a simple exhaust gas recirculation (EGR) system, both of which have a cost benefit
- A fuel system modified to suit DME.
Isuzu finds that DME is especially attractive in countries with Euro 3 standards, where the equivalent diesel engine leads to higher emissions; EAS is infeasible for such high-sulfur fuels. A DME engine with a fitted EGR has similar characteristics to a compressed natural gas (CNG) vehicle from a CO2 perspective, with higher thermal efficiency. Its distance and time-to-fill ratio is four times higher than CNG, and even better than diesel.
Isuzu has been working with Japan’s Ministry of Land, Infrastructure, Transport and Tourism (MLIT) on the legislation of technical standards for DME vehicles. In 2015, notification was received for the modified application, noting that for DME, the measurement of particulate matter (PM) is no longer necessary. Isuzu is expecting to enhance its DME test fleet in service to garner further support for the fuel.
DME’s qualification for alternative fuel vehicle financial incentives by Washington State was the first known legislation in the US that includes DME in its definition of those alternative fuels qualifying for financial incentives or special treatment. This is an important acknowledgement of DME’s relevance to businesses and governments that are eager to increase the use of clean alternative fuel vehicles.
California’s legalization of DME for use as vehicle fuel is the latest milestone for the growing DME industry. DME became the first biogas-based fuel to receive approval from the US Environmental Protection Agency (EPA) for inclusion under the Renewable Fuel Standard, making it eligible for valuable Renewable Identification Numbers (RINs) credits based on EPA findings that the fuel achieves a 68% reduction in GHG.
Other significant milestones include the US Department of Energy’s confirmation of DME’s status as an alternative fuel, as set forth in the Energy Policy Act of 1992, the publication of specifications for DME fuel by both the ISO and the ASTM.
Comparative assessment of DME and other fuels. The most recent version of the comprehensive European well-to-wheels analysis of automotive fuels and powertrains published last year, Well-to-wheels analysis of future automotive fuels and powertrains in the European context, includes comparisons of well-to-wheel energy use and GHG emissions for synthetic diesel and DME pathways from gas-to-liquid (GTL), coal-to-liquid (CTL), and biomass-to-liquid (BTL) production processes. A broad range of differentiation within the pathways exists (e.g., pipeline or remotely sourced natural gas; farmed or waste wood; production with or without CCS).
The report states that DME can be produced from natural gas or biomass with lower energy use and GHG emissions than other GTL or BTL fuels. With DME as the only product, the yield of fuel for use for diesel engines is high. The analysis estimates that the most likely feedstock in the short term is natural gas, but coal or wood can also be envisaged. Should DME become a major fuel, future plants would most likely be similar to GTL plants, i.e., large and located near a major gas field. Carbon capture, use and storage (CCUS) processes could be conveniently applied in this case, particularly because CO2 must be separated in the synthesis process and is, therefore, already “captured.” However, because DME synthesis is simpler than FT technology, smaller plants located in Europe and fed with imported gas can also be envisaged.
With increasing global interest in clean fuels, the market is seeing court cases backed by public advocacy groups, such as in the UK and the Netherlands, as well as increasing interest from governments in moving away from diesel reliance. Where project scales are economic and gas prices competitive, it is expected that DME can be introduced as a fuel at a discount to current diesel prices. With a relatively straightforward manufacturing process and low operational costs, DME is the type of fuel that can be introduced in the market in a hub-spoke system with cornerstone projects being built in key locations (such as California, Texas and around the Bakken and Marcellus shale formations in the US), and then gradually interlinked.
It is believed that by creating the right regulatory and legislative framework for the adoption and usage of DME, governments can support the collective ambitions of upstream companies to see a route to market-priced gas sales and to improve air quality. Gas-rich countries can easily act as large producers of DME, with added opportunities to export to adjacent markets that require either LPG feedstock or an alternative to diesel. Similar to LPG, but with some small modifications required, the logistical costs will be low. GP
 |
Rohit Vedhara, a commercial graduate from the UK, worked for BP in a number of refining, trading and strategy positions for over 20 years before forming Aum Energy in 2010 to help bridge the gap between innovative low-carbon technology solutions, finance and the market. Current projects include coal-to-biogas conversion using a process that links biotech with coal fields and gas production; partial upgrading for heavy crude oils using high-pressure/high-temperature nozzles; and low-cost biomass-to-power for Africa.




Comments