Mitigate corrosion in sweet gas amine units
J. Kittel, IFP Energies nouvelles, Solaize, France; M. Bonis, Total, Pau, France; and G. Perdu, Prosernat, Paris La Défense, France
An extensive review of acid gas removal units covering five decades and more than 120 amine units showed that units treating sweet gases (i.e., those with CO2 only) presented the worst risks of corrosion.
In some extreme cases in diethanolamine (DEA) units, a fairly uniform corrosion in an active mode was observed on stainless steel (SS) grades, such as AISI 410 and 304L, associated with strong scaling of the heat exchangers by corrosion products. A detailed analysis of these corrosion phenomena, complemented by laboratory investigations, enabled an understanding of this corrosion and the development of updated design criteria for sweet amine units.
Fouling and corrosion in sweet DEA units. A review performed in 20041 showed that corrosion in sour units (i.e., those with CO2 and H2S) was well understood and easily resolved by appropriate design and operating practices. Alternatively, unprecedented corrosion damages were reported for DEA units treating sweet gas (i.e., those with CO2 only).
Appropriate mitigation strategies were proposed in a 2008 article.2 However, there was no clear understanding of the increased corrosivity of sweet vs. sour DEA systems, or for DEA vs. methyldiethanolamine (MDEA) or formulated MDEA.
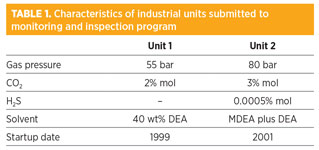
A program was then launched to understand the industrial feedback by laboratory studies. Two industrial units were selected, the main characteristics of which are summarized in Table 1.
 |
Results from DEA Unit 1. This unit experienced severe corrosion and scale problems, mainly:
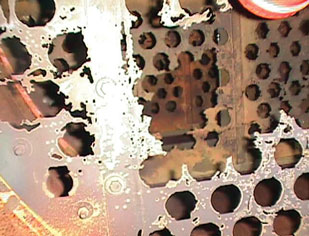
- Perforation and loss of mechanical integrity of the AISI 410 trays at the bottom of the absorber and at the top of the regenerator after less than 27 months of operation (Fig. 1)
- Uniform and well-adhering deposits with a thickness above 1 mm on the top trays of the regenerator
- Iron carbonate deposits in the amine/amine heat exchanger (Fig. 2).
 |
|
Fig. 1. Uniform corrosion of 13% Cr top trays of the |
 |
|
Fig. 2. Iron carbonate scale growth (DEA Unit 1). |
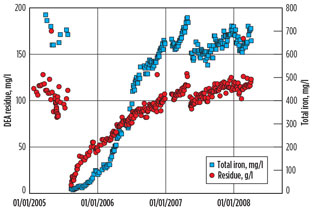
The AISI 410 trays were replaced with AISI 316L trays, and the unit has been operated without specific corrosion troubles for more than eight years. However, this unit continued to suffer strong FeCO3-like scaling in rich/lean exchangers, at the rate of 150 kg/yr. The evolutions of iron (Fe) dissolution and of amine degradation are summarized in Fig. 3. DEA residue corresponds to amine degradation products.
 |
|
Fig. 3. Summary of the evolutions of Fe dissolution and of |
From summer 2005 (DEA renewal) to winter 2007, dissolved Fe continuously increased from 0 mg/l to approximately 700 mg/l, with an average dissolution rate close to 2 mg/l/d, corresponding to a low average corrosion rate of wet carbon steel parts below 0.1 mm/yr.
Once saturation was reached, all Fe produced by corrosion was precipitated and recovered mainly in the amine/amine exchanger distribution boxes and inlet strainers. Considering a solvent inventory of 130 m3 and a dissolution rate of 2 mg/l/d, the calculated FeCO3 precipitation is in good agreement with approximately 150 kg of deposits accumulated each year.
DEA degradation follows the same trend as dissolved Fe. This is a strong indication that Fe complexation could be the result of reaction with DEA degradation products. The natural solubility limit of Fe in water saturated with bicarbonate is extremely low (below 1 mg/l). The presence of a strong chelating agent is, therefore, mandatory to reach 700 mg/l.
Results from MDEA/DEA Unit 2. The metallurgy of the main pieces of equipment is as follows (unit is located offshore):
- Absorber, flash drum, reflux drum: carbon steel (CS) and SS cladding
- Regenerator: CS and SS cladding at three top trays
- Rich amine lines: SS
- Lean amine lines: CS.
This unit did not present any corrosion issues.
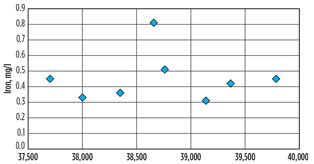
Dissolved Fe, measured over a six-year period, was below 1 mg/l, much lower than levels encountered in DEA Unit 1 (Fig. 4). It cannot be solely explained by the limited use of carbon steel or by a presumably lower corrosion rate of carbon steel (measured dissolved Fe would correspond to a corrosion rate lower than a few µm/yr). More probable is that the chelating tendency of MDEA or of MDEA degradation products is far less than that of DEA, and all dissolved Fe immediately precipitates.
 |
|
Fig. 4. Dissolved Fe, measured over a six-year period, was |
Laboratory investigations: Experimental method. The objective of the lab investigation was to reproduce the main trends of both industrial units and to provide meaningful information on the mechanisms involved and on the governing parameters.
The laboratory-prepared amine solutions were compared with an industrial solvent sample available from DEA Unit 1 and used as a reference. They were artificially degraded to better represent plant actual working conditions, by exposition for one week at 140°C and 0.5 mol/mol CO2 loading (α) with 60 g of Fe filling in the reactor, and then filtered.
Corrosion was studied in hot rich conditions, representative of a regenerator inlet, and then in hot lean conditions encountered at a stripper outlet.
All experiments were carried out in an autoclave made of a proprietary nickel-molybdenum-chromium-wrought alloy. Corrosion coupons were made of AISI 1020, AISI 410, 304L and 316L steel.
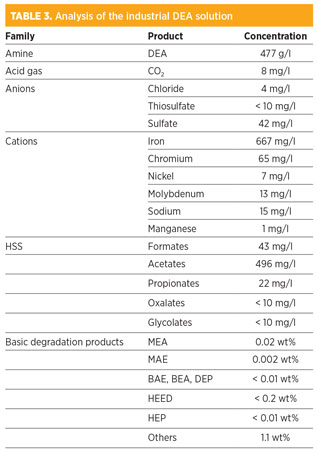
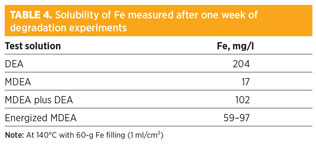
Artificial degradation step. Table 3 shows a complete analysis of the industrial solution before it was used in the laboratory for corrosion tests. Fe concentration in synthetic DEA solvent was found to be approximately one-third that of the industrial DEA solvent. Even though the Fe concentration is lower in MDEA-based solvents than in a DEA solvent, it was much higher than those measured in the industrial MDEA/DEA unit 2 (i.e., < 1 mg/l). The presence of large sections of SS in Unit 2 may explain the result (Table 4).
 |
 |
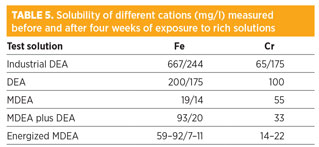
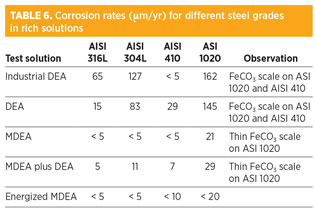
Rich amine experiments. A study determined the cations content and corrosion rates of steel coupons exposed to industrial DEA and to amine solvents submitted to artificial degradation (Tables 5 and 6).
 |
 |
Iron solubility in rich conditions is strongly enhanced in DEA, in comparison to MDEA solutions. Iron solubility decreased between the start and the end of all tests. Several conclusions can be drawn:
- Iron solubility reached after the degradation step at 140°C is greater than at a lower temperature
- Dissolved iron levels after the test at 110°C in rich conditions represent a true solubility limit
- All dissolved iron produced by corrosion during the experiment produced iron carbonate precipitates.
The highest corrosion rates were found with DEA solutions.
For AISI 316L and AISI 304L, measurable weight losses were obtained in DEA solutions, with values above 0.1 mm/yr for 304L in the industrial DEA. This illustrates the ability of degraded DEA to weaken the protectiveness of the passive layer of austenitic SS, as already inferred from high concentrations of dissolved Cr.
Corrosion rates of 304L and 316L were close to, or below, the detection limit in all MDEA solutions. Although chromium levels were lower in these solutions, the values remained too high to be compatible with perfect passivity. Therefore, low corrosion rates of austenitic SS might also be explained by a considerably lower potential corrosivity of MDEA-based solvents. MDEA plus DEA shows an intermediate behavior, while pure MDEA or energized MDEA demonstrate equally good performances.
In comparison to stainless steels, corrosion rates of CS in DEA solutions might seem surprisingly low. A relatively adherent FeCO3 scale had formed at the surface of the coupons, providing some protection against corrosion (Fig. 5); this occurrence is directly related to the hydrodynamic conditions of the laboratory experiments. On the contrary, in places where the plant faces high velocities, turbulent flow and effects of flash gas, erosion of iron carbonate deposits results in rapid active corrosion.
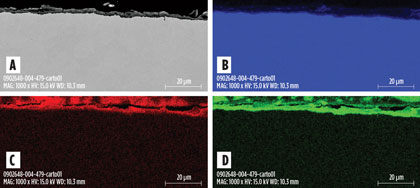 |
|
Fig. 5. Cross-section SEM analysis of AISI 1020 coupon after the test in hot rich |
AISI 410 presents an intermediate behavior. Although its corrosion rate remains low in DEA solutions, visual observation and scanning electron microscopy (SEM) of a cross-section analysis revealed an active corrosion behavior with the formation of a protective iron carbonate layer very similar to the one observed on CS coupons (Fig. 6). It is, therefore, assumed that AISI 410 presents an active corrosion in rich DEA, only balanced by laboratory conditions favorable to the formation of a protective scale.
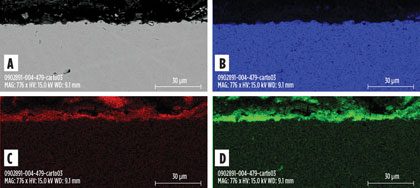 |
|
Fig. 6. Cross-section SEM analysis of AISI 410 coupon after the test in hot rich |
In plant conditions with erosion, rapid active corrosion is expected. In comparison, the low corrosion rate of AISI 410 in MDEA solutions was not attributed to iron carbonate, but to a good passivity in MDEA solutions or to a low potential corrosivity of this solvent.
This hypothesis was confirmed by electrochemical measurements in hot rich DEA for AISI 316L and AISI 410 (Fig. 7)—the corrosion potential of AISI 316L remained relatively high (> –0.5 V vs. Ag/AgCl) corresponding to the passive region, while AISI 410 showed a strong decrease of corrosion potential in the active corrosion potential range.
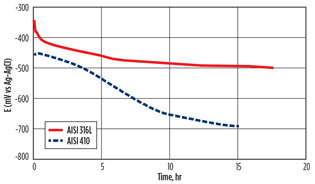 |
|
Fig. 7. Evolution of the corrosion potential of different SS |
These results present good correlation with industrial feedback. In particular, the quick active corrosion of AISI 410 trays in the hot rich sections of the absorber and the stripper of the DEA units can be explained by the insufficient resistance of the passive layer of AISI 410, allowing rapid active dissolution, with FeCO3 layer collapse under the high liquid/gas local velocities supported by the tray deck. Even the active dissolution of AISI 304L in highly degraded DEA could be reproduced in the laboratory. Additionally, these experiments also justify the choice of AISI 316L as replacement material for the trays of DEA Unit 1.
The lower corrosivity of the MDEA-based solvent on a more extensive use of corrosion-resistant alloys (CRAs) explain the good resistance to corrosion in MDEA/DEA Unit 2.
Lean amine experiments. As shown in Tables 7 and 8, AISI 316L and AISI 304L presented excellent corrosion resistance, confirming their ability to maintain passive state. AISI 410 also presented good results in both DEA and MDEA solutions. Contrary to tests with rich amine, no iron carbonate deposit was observed on AISI 410 coupons, suggesting that corrosion resistance in lean conditions is also associated with good passive behavior.
 |
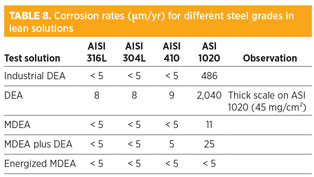 |
CS exhibited extremely high corrosion rates in lean DEA. This is probably a consequence of a residual corrosivity of the solutions due to an insufficient stripping of CO2, and to conditions not prone to form an FeCO3 protective deposit. This is due to the controlling complexing power of DEA solutions associated with high corrosion rates. On the contrary, all tests in lean MDEA solutions gave good results for CS because of a naturally lower corrosivity of the lean MDEA or formulated MDEA.
Discussion: Corrosion in sweet amine units. Laboratory experiments explained most corrosion and fouling problems encountered in the DEA sweet gas unit. The high level of iron solubility suggests a high concentration of chelating species, capable of complexing metals.3 This complexing power is higher in the industrial DEA solution, which confirms the role played by DEA degradation products.
Complexation induces depassivation of SS and allows rapid active corrosion. This was confirmed in the laboratory and in the field for AISI 410, and even for AISI 304L in strongly degraded solution. For low-alloy steels, complexation also delays precipitation of iron salts, such as FeCO3, providing corrosion protection. The good corrosion resistance of AISI 316L in DEA experiments confirms the positive field experience.
The experimental study showed that iron solubility depends on CO2 loading, being four to eight times higher in lean conditions. The iron carbonate formed in the rich section is precipitated out of the lean solvent. The cooling that occurs in the amine/amine heat exchanger further increases the precipitation of salts. This explains the local precipitation of iron carbonate in the hot side of the exchanger, as observed in DEA Unit 1.
Corrosion rates of MDEA on both SS and CS remained low compared to those of DEA. It is obvious that the potential corrosivity of MDEA or formulated MDEA is much lower than that of DEA. Furthermore, iron solubility is much lower. One major difference between MDEA and DEA is the possibility to form amine carbamate with secondary amine (DEA), while the only reaction product of tertiary amine (MDEA) and CO2 is bicarbonate ion. It is known that metals can form strong complexes with carbamate, as observed in the urea synthesis with ammonium carbamate. The presence of bicarbonate in rich MDEA reduces levels of dissolved iron.
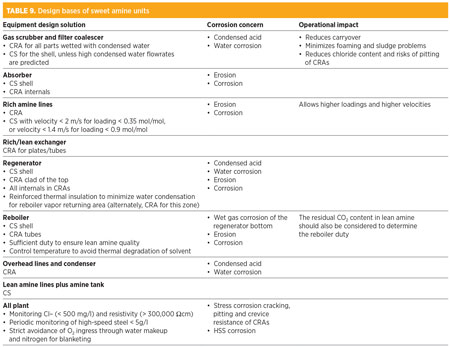
Updated design criteria for sweet amine units. Several design criteria incorporate the requirements of most operators—i.e., energy efficiency, reliability and flexibility to gas composition. They promote the use of SS instead of CS on all corrosion-susceptible locations (but only on those), combined with the use of high solvent loadings of any generic amines without the addition of corrosion inhibitors. The appropriate selection of CRAs gives the opportunity to solve all erosion/corrosion failures related to high velocities and high loading. The extended use of SS also has the advantage of minimizing inspection and maintenance costs.
For sweet units, DEA should be avoided if CS is used. The use of DEA requires the extensive use of AISI 316L. On MDEA or energized MDEA units, the risk of corrosion of CS by the solvent should not be considered an issue while any type of alloy gives trustable solutions for internals (Table 9).
 |
Takeaway. In sweet service, DEA solutions present severe risks of losses of material and corrosion of parts subject to turbulences. High iron solubility, especially in degraded solutions, suggests a high concentration of chelating species, resulting in poorly protective corrosion scales. Potential corrosivity is enhanced in hot rich conditions, and the lower grades of SS, such as AISI 410 and even AISI 304L, lose passivity and are subject to active dissolution. AISI 316L presents good corrosion resistance.
These steels also present specific risks of localized fouling. A significant difference in iron carbonate solubility exists between rich and lean conditions, and high dissolved iron concentration causes localized precipitation in hot amine sections, such as the exchanger.
In sweet service, MDEA solutions present a much lower chelating tendency. Also, mixtures of MDEA and DEA (at low activation grades) behave closely to MDEA. Furthermore, the energized MDEA features similarly low corrosivity to pure MDEA in sweet service for CS.
The following rules are still considered as the best long-term answers to the needs of most operators:
- No limitation to the amine loading for corrosion reasons to prioritize the process cost efficiency
- Use of generic amines and blends of generic amines are possible and preserve the flexibility to suppliers and to solvent changes
- Use of CRA on all corrosion-prone areas to preserve the reliability and flexibility of the unit to changes in flowrates, process conditions and type of amine
- Good operating practice, including avoidance of oxygen ingress, limit of thermal stress and thermal degradation, and periodic monitoring of solvent degradation products. GP
LITERATURE CITED
1Bonis, M. R., J. P. Ballaguet and C. Rigaill, “A critical look at amines: A practical review of corrosion experience over four decades,” 83rd annual Gas Processors Association convention, San Antonio, Texas, 2004.
2Kittel, J., M. R. Bonis and G. Perdu, “Corrosion control on amine plants: New compact unit design for high acid gas loadings,” Sour Oil & Gas Advanced Technology Conference (SOGAT), Abu Dhabi, UAE, 2008.
3DuPart, M. S., P. C. Rooney and T. R. Bacon, “Comparing laboratory and plant data for MDEA/DEA blends,” Hydrocarbon Processing, February 1999.
4Fleury, E., “Etude de la corrosion des aciers inoxydables dans les procédés de traitement des gaz par les amines,” Thèse de l’Université de Bourgogne, Dijon, France, 2010.
J. Kittel holds a materials engineering degree from INSA Lyon in France, and a PhD in electrochemistry from Paris 6 University in France. Since joining IFP Energies nouvelles in 2005, he has worked as a research engineer in the electrochemistry and materials department. His fields of expertise cover corrosion aspects in most environments of oil and gas production, including H2S cracking, amine units and CO2 capture. He is the author of more than 20 articles in scientific journals and 40 communications.
Michel Bonis is a corrosion expert at Total SA. He received his PhD in 1982, having completed his thesis on sweet gas corrosion in the oil and gas industry.
He has worked in the corrosion area since that time, at Elf Aquitaine and then Total. His expertise is in managing internal corrosion caused by production and process fluids. He has published more than 60 papers.
Gauthier Perdu is the deputy process director at Prosernat and an expert in
gas sweetening technologies. He graduated from ENSIGC Toulouse in France with
a degree in engineering and a post-graduate certificate in chemical engineering.
He started his career as a process engineer at Technip and joined Prosernat in 2002 to work with AdvAmine technology. Mr. Perdu has authored more than 12 industrial articles about the operation and design of gas sweetening unit technologies.




Comments