Improve amine unit efficiency by optimizing operating conditions
D. Satyadileep, A. S. Berrouk and C. J. Peters, The Petroleum Institute, Abu Dhabi, UAE;
and A. A. Aziz, Abu Dhabi Gas Industries Ltd., Abu Dhabi, UAE
How can the efficiency of an amine gas sweetening unit be improved without additional capital investment? Operation at optimum conditions is the first key to enhance the performance of the unit, as revealed by a case study performed on a commercial gas plant using a kinetics-based simulation analysis.
Lower amine temperature provides a significant reduction in solvent circulation rate, steam consumption rate, pumping duty and dehydration unit load while posing no risk of hydrocarbon condensation or hydrate formation. Also, higher amine strength is best suited for the unit under study, as it results in potential savings of operating costs without increasing the risks of corrosion and fouling.
In recent years, there has been emphasis on improving amine gas sweetening plant efficiency due to the increased exploitation of highly sour gas fields. This triggers the development of several optimization techniques to maintain the profitability of gas plants. Schemes involving operational changes alone are most preferred, due to the simplicity and flexibility in restoring plant operations and for the low retrofitting costs attached to them.
To this end, a case study was performed for a commercial gas sweetening unit to analyze the effect of various operating conditions on the plant efficiency. Operating data for this unit was used to quantify the impact of tuning various process conditions and to provide guidelines to achieve optimum operation of the amine sweetening unit.
Process simulation model
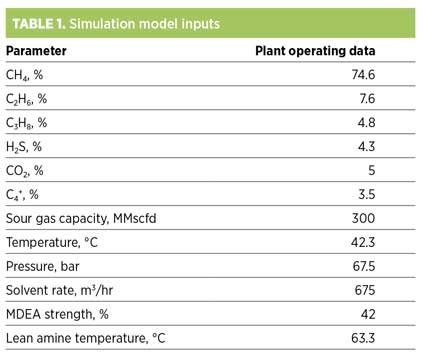
To investigate the effect of various process conditions, a process simulation model is developed for a commercial amine sweetening unit. Table 1 provides the plant operating data used to develop the simulation model.
The process simulation model developed using a commercial process simulator follows an “ideal-stage” approach, coupled with kinetic modeling for CO2. With an ideal-stage model, each stage is assumed to reach thermodynamic equilibrium, and a real column is modeled by determining the number of ideal stages that yields the same performance as the real column.
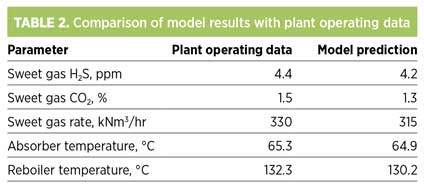
The kinetics model accounts for varying absorption rates of the relatively slow absorption of CO2, which is kinetically limited. Residence time for this calculation is computed based on column hardware and total number of trays. A customized Peng-Robinson model is used as the thermodynamic method in this study. The simulation model predictions are validated against plant operating data, as shown in Table 2.
Result and analysis
Optimum performance of the amine sweetening unit depends on proper tuning of the operating conditions. These generally include feed gas pressure and temperature, lean amine temperature and amine concentration. However, for the plant in this study, raw natural gas is directly fed to an absorption column without being compressed or heated within the battery limits of the amine sweetening unit. Therefore, the operator cannot manipulate the feed gas pressure and temperature, as they are governed by the upstream sections. The scope of this study focuses on the analysis of the effects of lean amine temperature and amine concentration on the amine sweetening unit performance.
Lean amine temperature. Lean solvent temperature has always been a point of interest, as it is the only parameter available for controlling the absorber temperature. Low operating temperatures are generally preferred for better performance of the absorption column due to the increased acid gas absorption governed by vapor-liquid equilibrium. However, there are also detrimental effects on the absorber operation associated with low lean amine temperatures.
The optimum choice of lean amine temperature entering the absorber is not a straightforward exercise, and it should be handled carefully to ensure the right balance between maximum performance possible in terms of acid gas removal, and smooth operation. To this end, a thorough quantitative investigation of the impact of varying lean amine temperature on several operational parameters of the amine sweetening unit is required. These operational conditions are:
- Sweet gas H2S content
- Sweet gas CO2 content
- Foaming due to hydrocarbon condensation
- Hydrate formation
- Lean solvent pumping duty
- Sweet gas water vapor content.
Analysis is performed using the process simulation model that is developed based on the operating data of a commercially operating amine sweetening unit, as shown in Table 1.
The table shows that the operating temperature of the lean amine is 63.3°C. The sensitivity analysis was carried out by varying the lean amine temperature in the range of 63.3°C to 47.3°C. The lower bound of this range is dictated by the gas feed temperature, which is 42.3°C, as stated in Table 1.
The 5°C value is a difference that is widely recommended as a general rule for the gas feed and lean amine temperatures.1 The impact of varying the lean amine temperature in the range of 63.3°C to 47.3°C is detailed below for the abovementioned operation parameters.
Effect on H2S and CO2 absorption. Low lean solvent temperature is generally sought in gas sweetening plants due to its positive impact on H2S absorption driven by equilibrium. However, MDEA solvents are known to react kinetically with CO2, and any lowering of the lean amine temperature should reduce the reaction rate constant, thereby increasing the CO2 slip. For this reason, lowering the lean amine temperature may be ruled out due to its undesirable effect on sweet gas CO2 content. Therefore, for any change in lean amine temperature, the resulting additional CO2 slippage must not cause the sweet gas CO2 content to exceed the plant’s product specifications. If this cannot be ensured, it may cause significant reduction in the gas calorific value and/or dry ice formation in the downstream units.
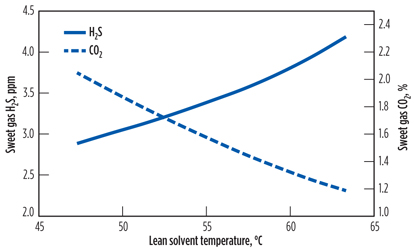
Fig. 1 shows the evolution of sweet gas H2S and CO2 contents as functions of lean amine temperature. As expected, H2S absorption is favored with lower lean amine temperature, while CO2 concentration in the sweet gas increases from 1.2% to 2%. The sweet gas profile for the lowest lean amine temperature (47.4°C) considered in this analysis is found to be compliant with the abovementioned plant requirements in terms of acid gas contents, despite the additional CO2 slippage.
 |
|
Fig. 1. Effect of lean solvent temperature on sweet gas H2S and CO2 contents. |
Therefore, reducing the lean amine temperature does not make objectionable changes in the sweet gas CO2. It is worth noting that a decrease in lean amine temperature results in a decrease in sweet gas H2S content from 4.2 ppm to 2.8 ppm. However, this decrease is not needed since the sweet gas H2S content is already below the plant specification.
In the following analysis, while decreasing the amine lean temperature, the sweet gas H2S content will be kept at 4.2 ppm by decreasing the energy requirement for stripping and decreasing the amine circulation rate.
Effect on foaming and hydrate formation. Low column temperatures promote undesired condensation of hydrocarbons. Indeed, accumulation of hydrocarbons in amine solvent often results in foaming that leads to poor column operation and to total shutdown, in some cases. Therefore, gas temperature must be sufficiently higher than the hydrocarbon dewpoint to prevent the occurrence of foaming.
Another ill effect associated with column temperature, but less frequently encountered, is hydrate formation, which can lead to catastrophic failure. Generally, hydrate formation temperature is far below the operating temperature. However, for columns operating at high pressures and low temperatures, hydrate formation temperature should be monitored.
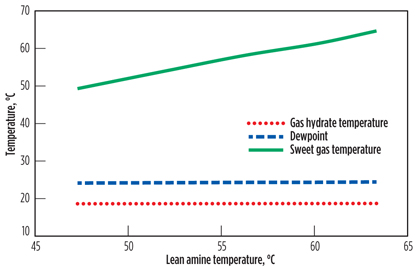
Hydrocarbon condensation temperature, hydrate formation temperature and gas operating temperature are plotted against lean amine temperature for the amine unit under study (Fig. 2). For the lowest lean amine temperature that can be considered, the sweet gas temperature is 25°C higher than the hydrocarbon dewpoint, which eliminates any possibility for the absorber to foam due to hydrocarbon condensation. Likewise, hydrate formation is obviously not a possibility, since the hydrate formation temperature is much lower than the hydrocarbon dewpoint.
 |
|
Fig. 2. Effect of lean solvent temperature on foaming and hydrate formation. |
Effect on lean solvent pumping duty. As the solvent viscosity increases with decreasing temperature, pumping loads are expected to be higher at lower lean amine temperatures. Therefore, pumping power must always be less than the available pumping power in the plant.
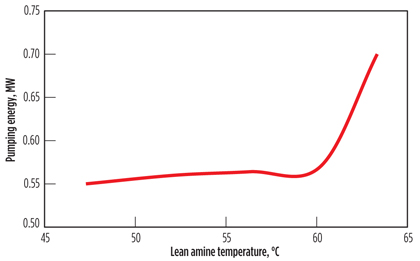
Fig. 3 shows the total electric load for pumping the lean solvent from the regenerator to the absorber. The pumping load is at its minima at the lowest lean amine temperature (47.4°C), which seems counter-intuitive. This is, in fact, due to the reduction in solvent circulation rate at a lower lean amine temperature, which is needed to meet the same acid gas removal level (4.2 ppm of H2S).
 |
|
Fig. 3. Effect of lean solvent temperature on solvent pumping energy. |
The reduction in amine circulation rate is more pronounced than the increase in solvent viscosity, thereby reducing the net pumping duty. It can be concluded that lowering the lean amine temperature will reduce the pumping cost, if the same sweet gas specifications are to be achieved.
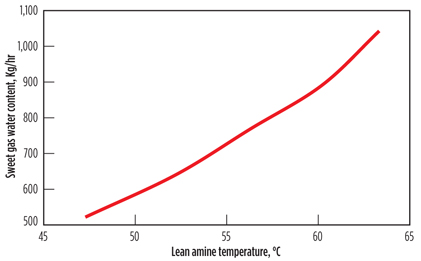
Effect on sweet gas water vapor content. It is well known that lower column temperature causes higher water vapor condensation in the absorber. This can have a potential benefit due to the reduced load on the dehydration unit downstream of the sweetening unit. Fig. 4 shows that sweet gas water vapor content decreases monotonically with lean amine temperature. As the lean amine temperature is decreased from 63.3°C to 47.4°C, half of the water vapor that should be carried by the sweet gas to the dehydration unit will condense and remain in the absorber. This results in a significant reduction in the operating cost of the dehydration unit.
 |
|
Fig. 4. Effect of lean solvent temperature on sweet gas water vapor. |
Summary of potential savings. Analysis of the effect of lowering the lean amine temperature on various operational parameters of the absorber results in potential benefits without disturbing the smooth operation of the absorber. Therefore, lower lean amine temperatures with a minimum of 47.3°C are recommended for the operation of the amine sweetening unit being studied.
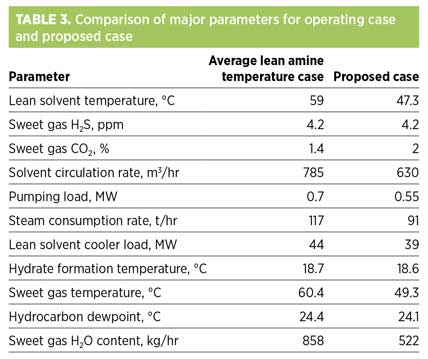
For this case, simulations have been performed and process optimization has been carried out while maintaining the sweet gas H2S concentration at the same level as that in the operating case. Also, simulations have been performed for the plant’s average lean amine operating temperature to quantify the savings resulting from the proposed case.
Table 3 presents a detailed quantitative summary of the major operational parameters for both cases. Besides the benefits discussed in the preceding sections, a significant reduction in steam consumption rate and solvent circulation rate can be observed for the proposed case. This is attributed to the increased acid gas holding capacity, which is due to the vapor-liquid equilibrium shift at low solvent temperatures. Also, the lean solvent cooler load is expected to be higher in the proposed case, as the outlet lean amine temperature is lower. However, this is outweighed by the lower solvent circulation rate, resulting in a lower solvent cooler duty, as shown in Table 3.
Therefore, this study concludes that lower amine temperature (47.3°C) provides significant reductions in solvent circulation rate, steam consumption rate, pumping duty and dehydration unit load while posing no risk of hydrocarbon condensation or hydrate formation.
Amine concentration. Amine concentration is of significant importance, primarily due to its impact on the sweet gas profile, since the former dictates the number of amine molecules that must be in contact with gas in the absorber. Industrial literature2, 3 has documented that poor choice of amine concentration leads to corrosion and fouling. Therefore, it is essential to maintain optimum amine strength to achieve a fine balance of the aforementioned associated effects.
Lower amine concentrations are generally considered to be an industrial precaution against corrosion. However, low amine concentrations, in combination with under-circulation of amine, lead to higher rich acid gas loading, thereby causing severe corrosion in the rich amine line.1 Also, low amine concentrations require a higher amine circulation rate and/or a higher reboiler steam rate to achieve sweet gas specifications in terms of acid gas removal.
On the other hand, higher amine concentrations often significantly minimize the operating cost as an implication of lower amine circulation rate and steam rate. However, higher amine concentrations promote corrosion rates on lean amine lines with time, due to acid gas flashing and the formation of layers of FeS and FeCO3.4 However, this risk can be mitigated with proper selection of solvent circulation rate and steam rate.
To summarize, the optimum choice of amine concentration can be made only through an investigation of its effect on several parameters:
- Sweet gas H2S and CO2 concentrations
- Steam consumption rate
- Amine circulation rate
- Rich amine loading (corrosion on rich amine lines)
- Lean amine loading (corrosion on lean amine lines).
To this end, the impact of amine strength on the preceding parameters has been analyzed through process simulations using different amine solvent concentrations:
- 42% (present operating condition)
- 45% (average lean amine strength in the plant)
- 47.5%
- 50% (plant design limit).
Simulations for the four cases were optimized to meet the operating value of sweet gas H2S concentration (4.2 ppm) to provide a candid comparison of the remaining parameters. Table 4 shows that sweet gas CO2 content is compliant with the requirements of the plant’s downstream section in all three cases. Rich and lean amine loadings are always less than the allowable limits (0.5 and 0.01, respectively1), which guarantees corrosion-free operations. Also, it can be concluded from the results that steam consumption and amine circulation rates significantly decrease as the amine strength increases, with the lowest values for steam use and amine circulation rate occurring at the highest amine strength (50%).
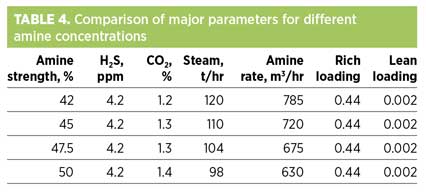
In summary, amine strength of 50% is best suited for the plant’s operating conditions, as it results in potential savings of operating costs without increasing the risk of corrosion and fouling.
Recommendations. Optimization of amine sweetening plants through operational changes has been a point of discussion due to the easy adoptability of changes without additional capital expense. This article investigated the impact on the plant performance of tuning the lean amine temperature and amine strength. Process simulations of an industrial gas sweetening unit are performed to analyze the possible benefits and detrimental effects that prevail as the two operating conditions are adjusted.
Lean amine temperature lower than the present operating condition is proposed as an optimum value based on simulation analysis. The proposed condition promotes H2S absorption with additional CO2 slippage, but within acceptable levels. This results in a reduction in steam consumption rate and solvent circulation rate to achieve the same sweet gas H2S specification.
Moreover, both the lean solvent pumping energy and the load on the dehydration unit decrease due to the reduction in the solvent flowrate and the higher degree of water vapor condensation in the absorber, respectively. The study also shows that the proposed operating conditions do not increase foaming tendency and hydrate formation.
The design limit for amine strength is recommended as the optimum value for the amine plant in this study. The process simulation model is optimized for this amine strength while meeting the desired levels of acid gas absorption. The results show that significant reductions in the steam consumption rate and the solvent circulation rate are achieved without increasing the risk of corrosion for the rich and lean amine streams. GP
Acknowledgment
This study was financially supported by The Petroleum Institute’s Gas Research Center in Abu Dhabi, UAE.
References
1Sheilan, M. H., B. H. Spooner and E. van Hoorn, Amine Treating and Sour Water Stripping, 3rd Ed., Amine Experts, Calgary, Canada, 2007.
2Patil, P., Z. Malik and M. Jobson, “Retrofit design for gas sweetening processes,” Proceedings of the 2006 IChem E Symposium, Series 152.
3Rechtien, R. and G. Duggan, “Identifying the impact of amine contamination on crude units,” Proceedings of 61st annual conference and exposition, Corrosion 2006, Houston, Texas.
4Pandey, M., “Process optimization in gas sweetening unit—A case study,” Proceedings of the International Petroleum Technology Conference, Doha, Qatar, 2005.
 |
Dara Satyadileep is a research engineer at The Petroleum Institute, with responsibilities including industrial research on gas processing technologies. He received a master’s degree in chemical engineering from the Indian Institute of Technology in Madras. He previously worked as a process engineer at General Electric for five years. His areas of interest include acid gas removal with physical and chemical solvents, sulfur recovery units, gas dehydration systems and gasification. One patent application has been filed in his name.
 |
Dr. Abdallah Sofaine Berrouk is an assistant professor at The Petroleum Institute’s chemical engineering department. He was awarded a PhD from the University of Manchester in the UK. He holds master’s degrees in engineering (energetics), research in solid mechanics, and oil and gas management. Dr. Berrouk’s expertise covers computational fluid dynamics, large eddy simulation, gas sweetening, aerosol dispersion and deposition, and computational flow modeling for chemical reactor engineering. Dr. Berrouk has published two books, three book chapters and more than 50 papers for peer-reviewed international journals and conferences.
 |
Alaa Abdul Aziz is head of the process engineering section of the technical services division at Abu Dhabi Gas Industries Ltd.’s Habshan gas complex. He received his bachelor’s degree in petroleum refinery engineering. He has 30 years of experience in the gas processing industry. His major areas of expertise include amine sweetening, gas dehydration, sulfur recovery units and natural gas fractionation.
 |
Dr. Cornelis Peters is a chair professor of The Petroleum Institute’s chemical engineering department. He earned his PhD from the Delft University of Technology in the Netherlands. Dr. Peters is an expert in the areas of thermodynamics, phase equilibria, ionic liquids, hydrogen storage, gas hydrates and CO2 sequestration. He is the author of more than 200 publications in peer-reviewed journals and is an editor or co-editor of six books and 13 book chapters. Two patents have been filed in his name.




Comments